Chemiluminescent Detection Assay Using Luminol - Crash Course
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
A chemiluminescent assay is a type of detection assay, or detection experiment for a specific molecule in your sample. This assay is based on chemiluminescence, which means production of light (luminescence) due to a chemical reaction (chemi).
The actual detection is based on this light emitted during the chemical reaction which forms the key part of the experiment.
The phenomenon of chemiluminescence is widely used in modern biochemical experiments to detect specific compounds – mostly proteins.
In Western blots and ELISA experiments, chemiluminescence represents a popular method of detection.
In this article we will first explore the principle of chemiluminescence.
Luminol, for example, is a common reagent in chemiluminescence detection assays. Using luminol as an example of a chemiluminescent substrate, we will demonstrate a typical chemiluminescent detection experiment.
Article Table of Contents:
What are the chemical requirements for chemiluminescence?
How is chemiluminescence utilized in biochemical experiments?
Luminol: a common chemiluminescent substrate
Chemiluminescence is a phenomenon wherein light (i.e., luminescence) is produced when a certain compound undergoes a chemical reaction in the presence of a catalyst, which is commonly an enzyme that can trigger the reaction.
Here is a classic example of a chemiluminescent reaction, as utilized commonly in actual lab settings. Catalyzed by the enzyme Horse Radish Peroxidase (HRP), Luminol undergoes a chemical reaction that produces light waves of wavelength 425nm.

Figure 1. Chemiluminescent reaction of luminol catalyzed by HRP enzyme.
In the next section we will take a detailed look at each component of a chemiluminescent reaction, learning why each is important.
Here are the chemicals absolutely required for chemiluminescence to occur:
The glow of fireflies is a classic example of chemiluminescence (bioluminescence) occurring in nature.
This light is produced during a biochemical reaction. The chemiluminescent substrate here is a substrate called luciferin that undergoes a reaction catalyzed by the enzyme luciferase, and the byproduct of this reaction is the light that glows.
In nature, chemiluminescence occurs in many other organisms. Since chemiluminescence here is associated with a biological entity, the technical term used is bioluminescence.
Bioluminescence is seen in various bacteria, fungi, snails, shrimps etc.
In most biochemical detection experiments the target molecule of interest is a specific protein that needs to be qualitatively assayed, and sometimes its levels are quantified as well.
In these experiments, researchers design an antibody that is specific and recognizes the protein of interest. This reagent is technically called a primary antibody.

Figure 2: First step of the chemiluminescent assay: primary antibody recognizes and binds to the target protein in the sample.
When it comes to an experimental setup, a surface-membrane is used for Western blots, while a well-plate is used for ELISA. The membrane or well will contain a protein mixture, which also contains your protein of interest.
Researchers then treat the membrane or well with a primary antibody to ensure that the actual target molecule is detected properly.

Figure 3. How a primary antibody detects a target protein on the experimental surface. This surface maybe a membrane (as shown here) in case of Western blot, bottom of a well in case of ELISA or a slide, as with immunohistochemistry.
Now comes the issue of detecting the probe, which in this example is our primary antibody.
Just to clarify, we have our target protein that we’re searching for. And we have our primary antibody that is able to find it. But the challenge with molecular biology is that we still can’t see this, which is why we have our little detection issue.
For this, researchers use another antibody, a second antibody, rightly named a secondary antibody.
The secondary antibody detects the primary antibody bound to the target protein.
This secondary antibody is conjugated, or joined, to an appropriate enzyme that can catalyze a chemiluminescent reaction.
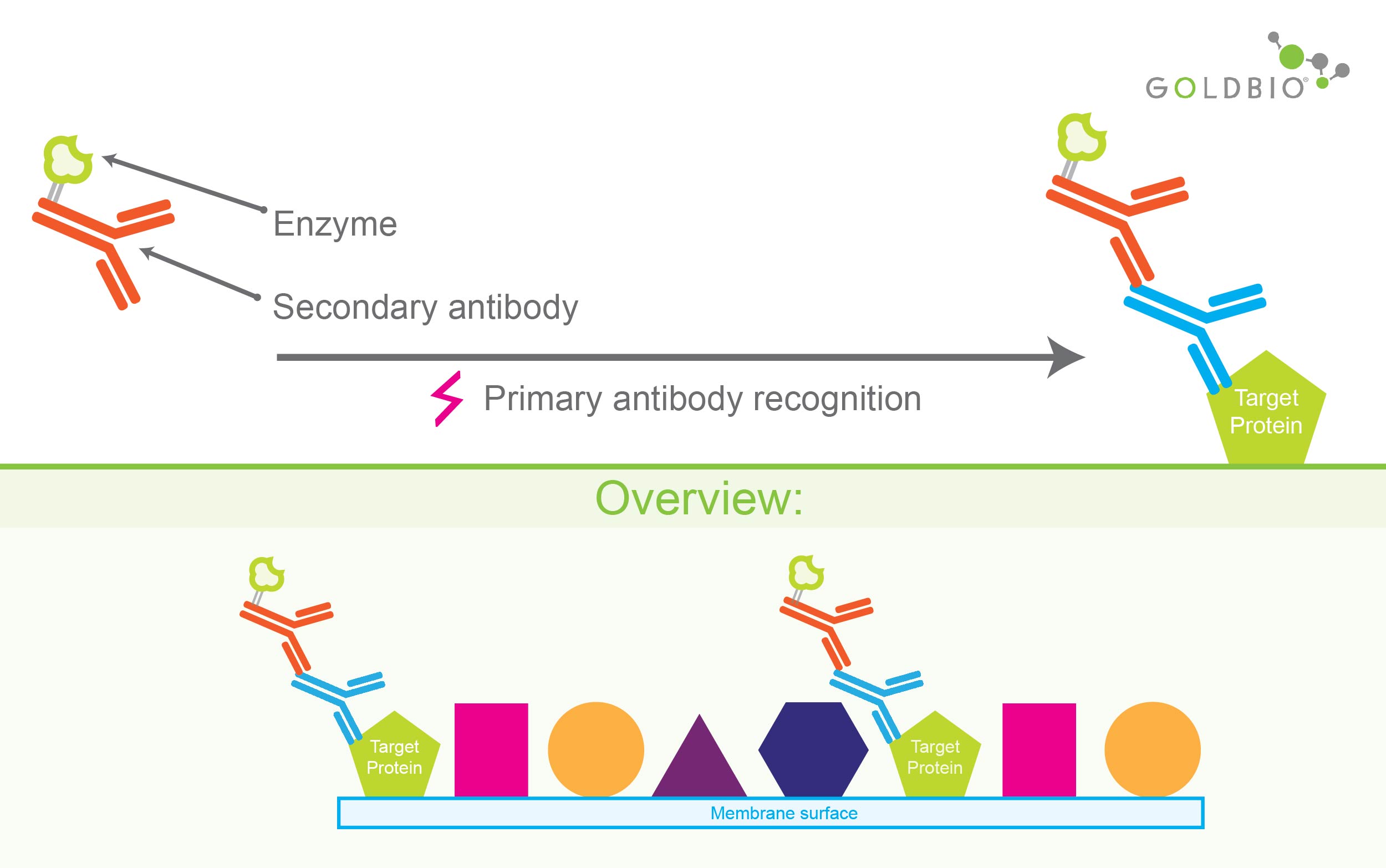
Figure 4. Secondary antibody detects and binds to primary antibody bound to the target protein. As shown in the top panel, the secondary antibody is conjugated with enzyme for catalyzing chemiluminescent reaction.
But for this enzyme to do its job, it needs a chemiluminescent substrate.
Adding this chemiluminescent reagent is the next step in the detection procedure.
But before this, the experimental surface is washed to remove unbound secondary antibody. This removes nonspecific and faulty detection.
After the primary antibody treatment, the experimental surface washed with a buffer before adding the secondary antibody.
The buffer wash ensures that unbound primary antibody molecules are washed away so that non-specific binding with secondary antibody molecules does not take place.
Similarly, after the secondary antibody treatment, there is another washing step before adding the substrate, to eliminate free-floating secondary antibodies from the experimental surface.
Washing steps are very important. They prevent false positive signals technically known as background noise.
This starts the chemiluminescence which is captured either on a phosphor or x-ray film or using specialized equipment capable of detecting and quantifying light across a spectrum of different wavelengths.
A typical chemiluminescent reaction commonly used in biochemistry experiments involves luminol as the chemiluminescent substrate and the enzyme horse radish peroxidase (HRP).
In the presence of an oxidizing agent, typically hydrogen peroxide, present in the reaction mix, HRP catalyzes the oxidation of luminol to 3-Aminophthalate.
This product compound exists temporarily in an excited state followed by transitioning to a stable lower energy state.
This transition of 3-Aminophthalate from a higher to a lower energy state is associated with the emission of light (chemiluminescence) waves of a wavelength of 425nm.
HRP-tagged antibodies and luminol are very popular for chemiluminescent detection in Western blots and ELISA experiments.
Popular features:
Other than biochemistry experiments in the laboratory, luminol is also widely used in forensic analysis for detecting traces of blood at crime scenes.
The catalyst for chemiluminescence in this case is hemoglobin – more specifically, the iron.
Iron, just like what we described with HRP and hydrogen peroxide, results in the oxidation of luminol – ultimately leading to the emission of blue light waves that can be detected and quantified at 425nm.
Karabchevsky et al. 2016. Tuning the chemiluminescence of a luminol flow using plasmonic nanoparticles. Nature.
Rose and Waite. 2001. Chemiluminescence of Luminol in the Presence of Iron(II) and Oxygen: Oxidation Mechanism and Implications for Its Analytical Use. Anal Chem. 73,24, 5909-5920

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...

Covalently conjugating a small molecule to an antibody’s surface is a process called antibody “labeling.” Labeling antibodies with small molecules such as biotin or fluorophores...

DNA gel electrophoresis is one of the most widely used analytical techniques in molecular biology, providing a simple and reliable way to separate DNA fragments...

Do you have a favorite restaurant that you love because you know exactly how great the experience is going to be? There are probably a...
