Chromogenic Substrates Overview
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
Chromogenic substrates aid in the qualitative and quantitative detection of enzymes or other proteins in biochemical experiments by producing a visible color (chromo) change.
The basic mechanism of chromogenic substrate-mediated detection is that the substrate produces a colored product that can be visually detected, when acted upon by a corresponding enzyme.
This enzyme may be the ultimate target of detection – like in enzyme assays.
Alternatively, the enzyme could be attached, or conjugated, to another molecule – mostly, an antibody – that detects and binds to the primary target protein in the sample.
The color change produced by cleavage of a chromogenic substrate can be visually deciphered through qualitative detection of the target enzyme or protein,
The intensity of the color change can also be measured. This is how quantitative detection of the target protein or enzyme is done using a chromogenic assay.
Common experimental techniques where chromogenic substrates are used are Western blot, ELISA, immunohistochemistry (IHC), enzyme assays and microbial detection procedures.
For more information about these common detection assays, you might want to take a look at our overview article about chromogenic assays.
This article is a snapshot of the chromogenic substrates sold by GoldBio, including the details of their chemical reactions and assays where they are useful.
Article Table of Contents:
DAB Tetrahydrochloride Hydrate
Each chromogenic substrate has a corresponding enzyme, which cleaves it to a colored product. This color is qualitatively or quantitatively assessed as a readout for the actual protein/enzyme that assay is targeting to detect.
Common enzymes in chromogenic assays are alkaline phosphatase (AP), beta-galactosidase (beta-gal) and horse radish peroxidase (HRP).
Table 1 lists a snapshot of the chromogenic substrates, the required corresponding enzymes, the color change and the type of experiments they are used in. This is followed by discussion on common chromogenic substrates and details of the reactions.
Each number in Table 1 represents a correspondence between substrate, color change and application. If no number is provided, then that characteristic could apply for all. For example, the substrate DAB (1) has a brown color change (1) and is often used in ELISA, western blot and IHC.
Table 1. Classification of the chromogenic substrates based on the enzymes and the experiments they are used in.
|
Enzyme |
Substrate |
Color change |
Application |
|
Horse Radish Peroxidase (HRP) |
|
|
ELISA, western blot and IHC
|
|
Alkaline Phosphatase (ALP) |
|
|
|
|
Beta-galactosidase (β-gal) |
|
|
Western blot, IHC, LacZ enzyme assay for screening transformed colonies
|
|
Alpha-galactosidase (α-gal) |
X- α-gal |
blue |
GAL4-based yeast two-hybrid interactions
|
|
Beta-glucuronidase |
|
|
Bacterial detection
|
3,3'-Diaminobenzidine (DAB) Tetrahydrocholride hydrate is a chromogenic substrate useful mostly in ELISA, Western blots and immunohistochemistry (IHC).

Figure 1. Chromogenic reaction using DAB as a substrate
This substrate, when acted upon by a peroxidase enzyme, produces an insoluble brown product that can be visually detected.
In actual experiments the conjugated peroxidase enzyme is most commonly Horse Radish Peroxidase (HRP).
Another HRP substrate is 3,3', 5,5-tetramethylbenzidine (TMB). This chromogenic substrate is used primarily in ELISA, western blot and sometimes in IHC experiments.
HRP oxidizes TMB to the blue colored product compound 3,3',5,5'-tetramethylbenzidine diamine.
The intensity of this blue coloration is measured spectrophotometrically at 650 nm for quantitative analysis of the target protein being assayed.
The higher the intensity of blue coloration, the higher the concentration is of the target protein in the sample.
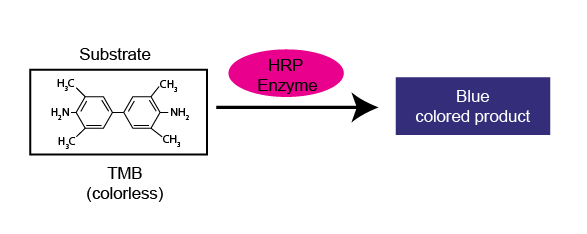
Figure 2. Demonstrates how TMB is utilized in a chromogenic Western blot experiment
The salt 5-bromo-4-chloro-3'-indolyphosphate p-toluidine (BCIP) and nitro-blue tetrazolium chloride (NBT) are commonly used together as a chromogenic substrate combination.
In experiments where BCIP-NBT is used as the chromogenic substrate, the corresponding enzyme conjugated to the probing antibody is alkaline phosphatase (AP).
The product formed is blackish purple that can be readily visible.
BCIP-NBT is suitable for being used in Western blots, IHC and can be added in microbiological solid media for detecting AP activity in the microbial culture.
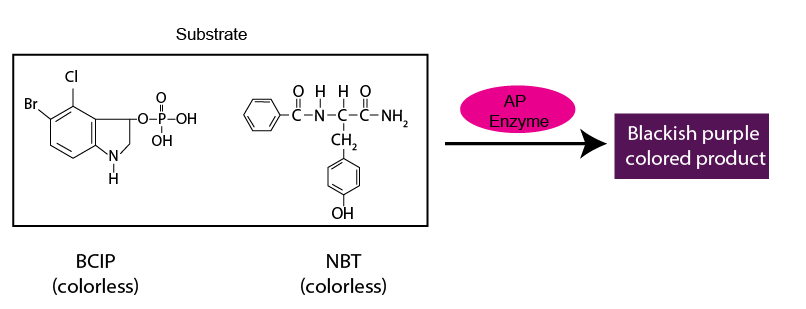
Figure 3. Chromogenic reaction using BCIP-NBT as the substrate.
When AP is the enzyme being assayed or is conjugated to the detector antibody, p-Nitrophenyl Phosphate (PNPP) is another chromogenic substrate option.
The product is yellowish in color, and it can be both visually detected (qualitative analysis) as well as spectrophotometrically measured quantitatively at 405 nm.
This forms the basis of how PNPP is used in quantitative ELISA experiments

Figure 4. PNPP being used as the chromogenic substrate in an ELISA. Please note the different degrees of yellow coloration in the different wells of the 96-well plate. Intensity of yellow color is a read out for the concentration of the target protein in the samples in the respective wells.
Chemically, X-Gal is 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (BCIG). This chromogenic substrate is commonly used in a variety of experiments.
The lacZ gene encodes the enzyme β-galactosidase enzyme. This gene and the encoded β-galactosidase is exploited as a readout or reporter for biological phenomena such as transcription from a promoter or translation level of a transcript.
Further, β-galactosidase is often used as a marker of gene cloning in bacteria, using a technique known as blue-white screening.
The substrate X-Gal is off-white in color, – a color that resembles solid media for bacterial culture.
When bacteria are cultured in a solid media plate with X-Gal added to it, the bacterial colonies not producing the enzyme β-galactosidase are whitish in color.
In contrast, the colonies producing β-galactosidase, are blue because β-galactosidase cleaves the chromogenic substrate X-Gal, producing a blue colored product called 5,5'-dibromo-4,4'-dichloro indigo.

Figure 5. Blue-white screening of bacterial colonies on a plate using X-gal as the chromogenic substrate.
In this way, you can distinguish which colonies produce β-galactosidase (blue colonies) versus those that do not (white colonies).
A few variants of X-Gal are commercially available. They are as listed below.
This is a halogenated indolyl-β-galactoside, a chromogenic substrate of the same enzyme (β-galactosidase) as X-Gal.
The colored product is a more intense blue, allowing easier detection. Bluo-Gal is used in blue-white screening, as well as in Western blots and IHC experiments.
The product of Magenta-Gal, when acted upon by β-galactosidase, is red rather than blue as in case of X-Gal. Because of the red product, screening with magenta-gal is called red-white screening.

Figure 6. Red-white screening of bacterial colonies on a plate using Magenta-gal as the chromogenic substrate
When 6-Chloro-3-indoxyl-β-D-galactopyranoside (salmon-gal) is cleaved by β-galactosidase, the product is a salmon color – hence the name salmon-gal.
Salmon-Gal is used in the same way as X-Gal is used for colony screening.
Just as X-Gal aids in detection of beta-galactosidase, X-α-Gal is the chromogenic substrate for detecting the enzyme α-galactosidase, leading to the formation of an insoluble blue product.
Beta-glucuronidase is naturally found in the bacterium E. coli. Exploiting this phenomenon, X-α-Gal is used to detect E. coli contamination – for example, in drinking water.
X-α-Gal is added to a sample of the water. If the sample is E. coli contaminated, a blue color change is observed.
Chemically, X-Gluc is the cyclohexylammonium salt of 5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid.
The corresponding enzyme for X-gluc is beta-glucuronidase. The product is chloro-bromoindigo – a deep blue precipitate. Spectrophotometrically, its intensity is detected at 615nm.

Figure 7. X-gluc being used in an experiment to detect E. coli contamination of water. If water is contaminated, X-gluc is cleaved to a blue colored product by a bacterial enzyme.
The enzyme beta-glucuronidase is encoded by the GUS gene – a common reporter in modern molecular biology experiments, akin to lacZ. This underscores the importance of X-gluc in gene cloning experiments.
Further, as with X-α-Gal, X-gluc is also useful in detecting bacterial contamination – of foods and beverages.
An alternative chromogenic substrate for beta-glucuronidase is p-Nitrophenyl-β-D- glucopyranoside (PNPG). It produces a yellow-colored product.
It is suitable for detecting microbes producing this enzyme – most commonly, E. coli.
Further, it is also a reagent for assaying the GUS reporter in molecular biology.

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...

Covalently conjugating a small molecule to an antibody’s surface is a process called antibody “labeling.” Labeling antibodies with small molecules such as biotin or fluorophores...

DNA gel electrophoresis is one of the most widely used analytical techniques in molecular biology, providing a simple and reliable way to separate DNA fragments...

Do you have a favorite restaurant that you love because you know exactly how great the experience is going to be? There are probably a...
