Fluorescence microscopy: A Basic Introduction
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
Fluorescence microscopy is a fluorescence-based imaging technique. The basic principle involves stimulating a fluorophore by light at a particular wavelength, resulting in light emission at a longer wavelength. The emitted light can be visualized with fluorescent microscopes.
Fluorescence microscopy is a common experimental technique nowadays. Furthermore, the field of fluorescence microscopy is undergoing fast growth. A working knowledge of this technique is essential to leverage these advancements.
Nevertheless, there is very limited centralized information readily available on this topic.
To address that gap, we will discuss the working principle of fluorescence microscopy, resources, experimental use-cases, drawbacks, and finally various advancements within this space.
Working principle of fluorescence microscopy
Sample preparation and visualization using fluorescent microscopy
How is fluorescence microscopy useful in research?
Different microscope techniques
Widefield microscopy or epifluorescence microscope
Fluorescence,a natural phenomenon, falls within a broader category of luminescence wherein a substance known as fluorophore absorbs light at high energy and
transitions to its excited state. Afterwards, it emits light at a lower energy
in the form of photons after a certain period of time.
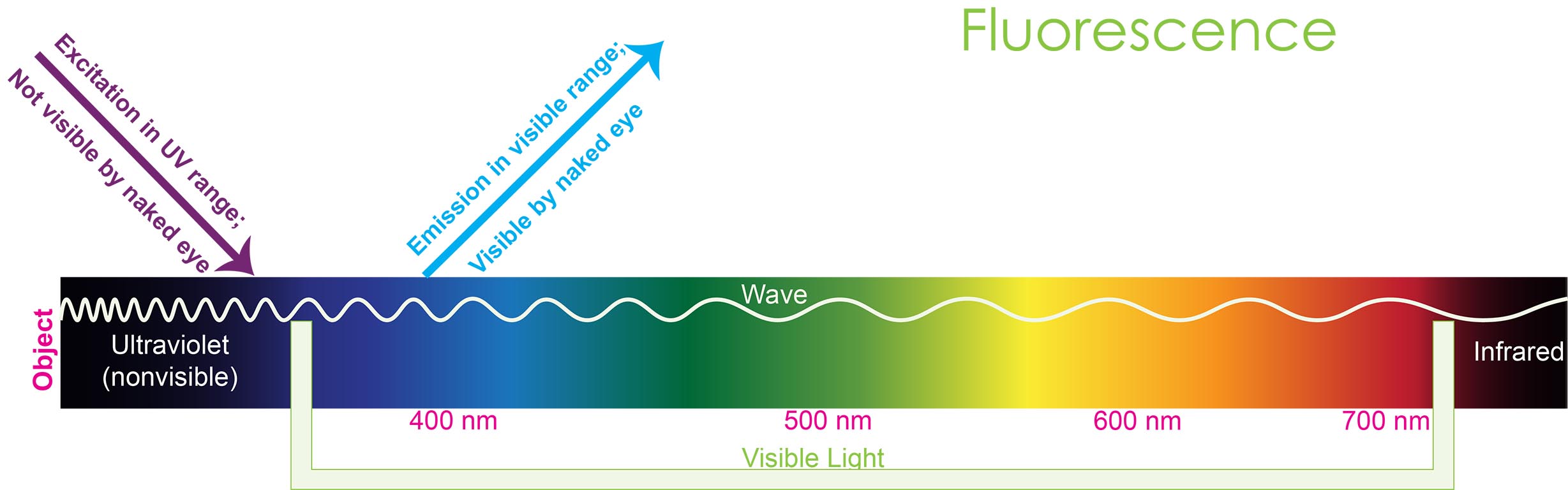
Figure
1.
Representation of fluorescence works.
A fluorescence microscope operates on the principle of exciting a fluorophore with light at a shorter wavelength, which causes it to emit light at a longer wavelength (see figure 1). The emitted light is then detected by the fluorescence microscope.
A fluorescence microscope is a highly effective imaging tool in the field of biology. This tool is used for spatial and functional characterization of biomolecules that exhibit autofluorescence in their native environment, such as in a cell/ tissue, as well as for molecules that are labeled with fluorescent molecules to emit fluorescence exogenously.
A fluorescence microscope generally has a light source, an excitation filter, an objective lens, a dichroic mirror and an emission filter.
Light source: Light sources can be gas arc lamps (mercury arc and xenon arc), LED in the case of conventional widefield microscopy, and laser light for advanced systems like confocal microscopes.
Excitation filter: Excitation filters select the light at a particular wavelength that will excite the fluorophore within the target specimen. This light illuminates a large portion of the specimen. When the fluorophores in the sample are illuminated with the proper wavelength, they emit fluorescent light at a lower wavelength.
Objective lens: The objective lens is a key component of the microscope that is used to magnify the specimen being observed. It is located close to the specimen, typically at the bottom of the microscope’s nosepiece. The target specimen on a glass slide is illuminated by the excitation wave passing through the objective lens. The quality of the objective lens is critical in determining the resolution and clarity of the image produced by the microscope.
Dichroic mirror: The dichroic mirror is also called a beamsplitter because it reflects the excitation waves towards the fluorogenic component in the sample and the transmitted emission waves from the sample towards the detector. For this, the excitation, emission filters and the dichroic mirror are strategically placed relative to each other.
Emission filter: This filter eliminates all wavelengths (including the excitation light waves) other than the one emitted by the fluorophore. This ensures that only the emitted light is transmitted for detection.
The filters and dichroic mirror are carefully selected to align with the excitation and emission wavelengths of the fluorophore. This helps to image the distribution of a single fluorophore molecule at a specific time, ensuring accurate visualization.
Thus, the microscope detects the image of only the fluorescent part of the specimen.
The resolution of a microscope is an important term used to describe its capability of distinguishing details in a specimen, which is determined by the minimum distance between two separate points in the specimen that can still be perceived as distinct entities.
The numerical aperture (NA) and wavelength of light are the determining factors for the resolution of optical microscope systems. The maximum limit of resolution achievable is approximately 200nm.
To visualize a sample with a fluorescent microscope, the target molecule or cellular component in your sample should be able to fluoresce.
Otherwise, they need to be labelled or tagged with fluorogenic molecules.
Fluorescent probes are compounds able to fluoresce. They are commonly referred to as fluorochromes or fluorophores.
One of the major breakthroughs is the development of genetically encoded fluorescent proteins, such as green fluorescent protein (GFP) and its derivatives. Fluorescence microscopy utilizes the fluorescent properties of fluorophores, for example, GFP, to label and detect target samples within various cellular components.
In immunofluorescence assays, these fluorescent
proteins can be fused to target proteins, enabling real-time visualization of
their cellular distribution and movement, as well as their interactions with
other proteins or cellular structures.
This has provided valuable insights into protein trafficking, protein-protein interactions, and protein dynamics in living cells which were previously difficult to study using traditional approaches.
To visualize the targeted molecule within the specimen, particular antibodies or proteins linked to the fluorophore are introduced into the cell. These fluorescently-tagged antibodies, in turn, bind to the target molecule in the sample that you want to visualize under the microscope.
The specimen is then exposed to the excitation wavelength and the resulting observation is performed using a filter that selectively permits the transmitted wavelength to pass while obstructing all other wavelengths.
Under the microscope, you see only your target biomolecule or cellular structure that is bound to the fluorescent-labelled probe. All other things and the entire background is dark. This allows very clear and crisp visualization of your target against a dark background.

In general, fluorophores are a diverse group of molecules including organic dyes such as fluorescein, rhodamine, cyanine. There are also inorganic ions like lanthanide ions, fluorescent proteins, and atoms. Table 1 lists some examples as well as the type of fluorophore they are.
To learn about how to label samples with fluorescent probes, refer to our article all about fluorometric detection assays.
Table1. List of fluorophores commonly used in scientific research.
|
Fluorophore |
|
Type of fluorophore |
Excitation wavelength |
Emission wavelength |
|
Cy2 |
|
Organic dye |
492 nm |
507 nm |
|
Cy3 |
|
Organic dye |
554 nm |
566 nm |
|
Cy5 |
|
Organic dye |
650 nm |
665 nm |
|
Alexa fluor 488 |
|
Organic dye |
499 nm |
520 nm |
|
Alexa fluor 633 |
|
Organic dye |
631 nm |
647 nm |
|
Alexa fluor 568 |
|
Organic dye |
577 nm |
603 nm |
|
BFP |
|
Fluorescent protein |
380 nm |
440 nm |
|
DAPI |
|
Organic dye |
359 nm |
457 nm |
|
eCFP |
|
Fluorescent protein |
435 nm |
476 nm |
|
eGFP |
|
Fluorescent protein |
489 nm |
509 nm |
|
eYFP |
|
Fluorescent protein |
514 nm |
527 nm |
|
Fluorescein FITC |
|
Organic dye |
494 nm |
517 nm |
|
Rhodamine TRITC |
|
Organic dye |
550 nm |
573 nm |
|
dTomato |
|
Fluorescent protein |
554 nm |
581 nm |
|
mCherry |
|
Fluorescent protein |
587 nm |
610 nm |
|
Texas Red |
|
Organic dye |
589 nm |
610 nm |
The selection of fluorophores depends upon specific application requirements, such as excitation and emission wavelengths, fluorescence intensity, stability, etc.
Also, the choice of fluorescent probes depends on which cellular component/ molecule you want to see. For example, DAPI is a fluorescent probe that binds to DNA.
Fluorophores help researchers study and manipulate the behavior of cells, organelles, and tissues with high sensitivity and specificity.
Fluorescent probes are rapidly advancing with immense potential in the biological and biomedical fields. These probes function as molecular reporters that emit fluorescence, enabling researchers to gather valuable information about molecular interactions within their immediate surroundings.
By analyzing the fluorescence signals, researchers can track the cellular localization of these probes in fluorescence microscopy images.
Fluorescence microscopy has revolutionized how we study cell physiology by visualizing the structure, function, and behavior of cells and tissues.
The discovery and continuous application of fluorescence microscopy has had a profound and long-lasting influence in the realms of biological and biomedical research. This technique helps researchers to
Fluorescence microscopy encompasses a broad range of configurations, ranging from a simple epifluorescent microscope to advanced setups like confocal systems that produce high-resolution images.
The most commonly used fluorescence microscope is the widefield microscope, which uses a wide range of light sources to stimulate fluorophores within the sample. The emitted light is then gathered by the objective lens and detected by a camera.
This is also known as epifluorescence microscopy – a basic form of fluorescence microscopy. “Epi” means the same; here the excitation and the emitted waves are transmitted through the same objective lens.
The widefield microscopy technique is generally easy to use and has a high processing speed.
Despite being user-friendly, it has limited contrast and resolution due to the out-of-focus fluorescence feature, resulting in blurred images.
Confocal microscopy produces high-resolution optical 3D images of cellular components.
It serves a wide range of functions including dynamic live cell imaging, detailed tissue morphology analysis, and determining protein interactions and expression patterns using co-localization studies.
In contrast to widefield microscopes, confocal microscopes generate sharp images of the precise plane of focus without background interference or background from other specimen regions.
This lets researchers easily visualize structures within even thicker objects without the blurring effects of fluorescent light from surrounding areas.
Brown. 2007. Fluorescence microscopy-avoiding the pitfalls. J Cell Sci. 120(Pt 10):1703-5. doi: 10.1242/jcs.03433
Drummen. 2012. Fluorescent probes and fluorescent microscopy techniques- illuminating biological and biomedical research. Molecules. doi: 10.3390/molecules171214067
Chalfie. 2009. GFP: lighting up light. Angew Chem Int Ed Engl. 2009;48(31):5603-11. doi: 10.1002/anie.200902040
Chalfie et al. 1994. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802-5.doi: 10.1126/science.8303295
Elliott. 2020. Confocal Microscopy: Principles and Modern Practices. Curr protoc cytom. 92(1). doi: 10.1002/cpcy.68
Ishikawa-Ankerhold. 2012. Advanced Fluorescence Microscopy Techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 17(4): 4047–4132. doi: 10.3390/molecules17044047
Jensen. 2012. Types of Imaging. An overview of fluorescence microscopy. American association for anatomy. https://doi.org/10.1002/ar.22548
Sanderson et al. 2014. Fluorescence microscopy. Cold Spring Harb Protoc. doi:10.1101/pdb.top071795
Slavik. 1996. Fluorescence probes. Fluorescence Microscopy and Fluorescent Probes. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1866-6_5

DNA gel electrophoresis is one of the most widely used analytical techniques in molecular biology, providing a simple and reliable way to separate DNA fragments...

Nickel affinity chromatography is a technique used to purify proteins that contain a polyhistidine tag (his-tag). Nickel chromatography is a popular choice for affinity purifications...

The best agarose for DNA gels depends on your application. Agarose LE is ideal for routine electrophoresis. Low melt agarose is best for DNA recovery,...

Making IPTG stock solution involves weighing out IPTG powder, pouring it into a conical tube or cylinder, and adding deionized water to the desired volume....
