Nourseothricin Overview
by Adriana Gallego, Ph.D.

by Adriana Gallego, Ph.D.
Nourseothricin is an aminoglycoside antibiotic and is a mixture of streptothricin D and F (>85%), and streptothricin E and C (<15%). This antibiotic is used for the selection of genetically modified Gram-positive and Gram-negative bacteria, yeast, filamentous fungi, protozoa, microalgae and plants during long-term experiments as nourseothricin retains >90% activity after one week under cultivation conditions.
This article presents an overview about nourseothricin structure, mechanism of action, bacterial resistance, applications, working concentrations according to the organisms and more.
In this article:
Nourseothricin structure and composition
Streptothricin backbone and structure
Nourseothricin mechanism of action
Nourseothricin resistance genes
Bacterial resistance to nourseothricin
Nourseothricin applications – research use, etc.
Considerations for nourseothricin working concentrations
GoldBio Nourseothricin sulfate advantages
Nourseothricin is a mixture of streptothricin D and F (>85%), and streptothricin E and C (<15%).
Therefore, its structure is rooted in the streptothricin molecular structure composed of a carbamulated D-glucosamine sugar, a streptolidine lactam, and a B-lysine homopolymer. The B-lysine group is what determines the type of streptothricin (D, F, E, C).
The structure of nourseothricin has three moieties:
The β-lysine homopolymer chain differs in the number of β-lysine residues from one to seven and giving rise to the different types of streptothricin from A-F and X.

The mechanism of action for nourseothricin is to inhibit protein synthesis by interfering with the mRNA translocation step, causing the misreading of the RNA molecule.
Nourseothricin is an aminoglycoside antibiotic. The mechanism of action for aminoglycoside antibiotics involves a two-step process.
In the first step, a self-promoted uptake involves the displacement of divalent cations such as magnesium and calcium from outer and inner membranes. Without these cations, the outer and inner membranes become more permeable. This in turn, elevates the antibiotic uptake.
After crossing both membranes barriers, the antibiotic enters the cytoplasm and targets the ribosome. In a second step, the aminoglycoside antibiotic binds to the A-site on the 16S RNA of the 30S bacterial ribosome hindering the normal translation process, which leads to mistranslated proteins in the cytoplasm. The accumulation of abnormal proteins accelerates cell death.
Nourseothricin is considered an aminoglycoside antibiotic because it also inhibits protein synthesis by interfering with the translation process and causes misreading of RNA molecules. However, nourseothricin does not have the typical aminoglycoside structure core of the dibasic aminocyclitol ring (commonly 2-deoxystreptamine). Instead, the lysine residue is responsible for the antibiotic effect.
Nourseothricin is a mixture of streptothricin D and F (>85%) and streptothricin C and E (<15%). It has been reported that streptothricin F causes errors in reading the genetic message.
In polypeptide synthesis directed by homopolynucleotides (regulatory elements at various stages of mRNAs life cycle), Streptothricin F stimulates the ribosome into incorporating the wrong amino acid during the translation process.
Also, streptothricin F inhibits the factors-dependent binding of aa-tRNA to the acceptor site of the ribosome with factors like EF-Tu. Additionally, streptothricin F strongly affects the translocation reaction, i.e., the transfer of peptidyl-tRNA from the acceptor site to the donor site (Haupt et al., 1978).
Furthermore, in a study, researchers found that streptothricin D (with three lysine moieties compared to one moiety present in strepthrocin F), exhibited a potent antibacterial activity against prokaryotic bacteria, such as E. coli, B. subtilis, and S. aureus, but not against eukaryotic cells such as S. cerevisiae and S. pombe.
Authors associated these results with an alternative resistance mechanism present in eukaryotic cells related with hydrolysis of the streptolidine lactam ring (Hamano et al., 2006).

The resistance genes for nourseothricin are Sat1, Sat2, Sat3 and Sat4. The sat genes code for streptothricin acetyltransferase proteins. Resistance is due to N-acetylation of lysine residue in the nourseothricin molecule mediated by the different streptothricin acetyltransferases in gram-negative bacteria.
The sat4 gene has been reported to belong to a gene cluster with other aminoglycoside resistance genes like aphA-3 and aadE which improve the bacterial resistance to nourseothricin (Derbise et al 1996; Wendlandt et al 2013).
The complete sequence for sat4 from Staphylococcus intermedius can be found here.
Bacterial resistance is due to N-acetylation of the β-amino group (C16) of the β-lysine residue in the nourseothricin molecule catalyzed by the enzyme acetyl coenzyme A: streptothricin acetyltransferase (in short ACSAT), causing a reduction of the antibiotic activity (Hahn, 1983).
To better understand nourseothricin inactivation, let’s take a closer look at the composition of commercially available nourseothricin.
Nourseothricin is a mixture of streptothricins D, F, C and E. The acetyltransferase synthesized by Streptomyces laoendulae acetylates the streptothricins at the β-amino group (C16) of the β-lysine residue, protecting the strain against its own antibiotic product (Ziihringer et al 1993). Below there is an example of inactivation of streptothricin F.
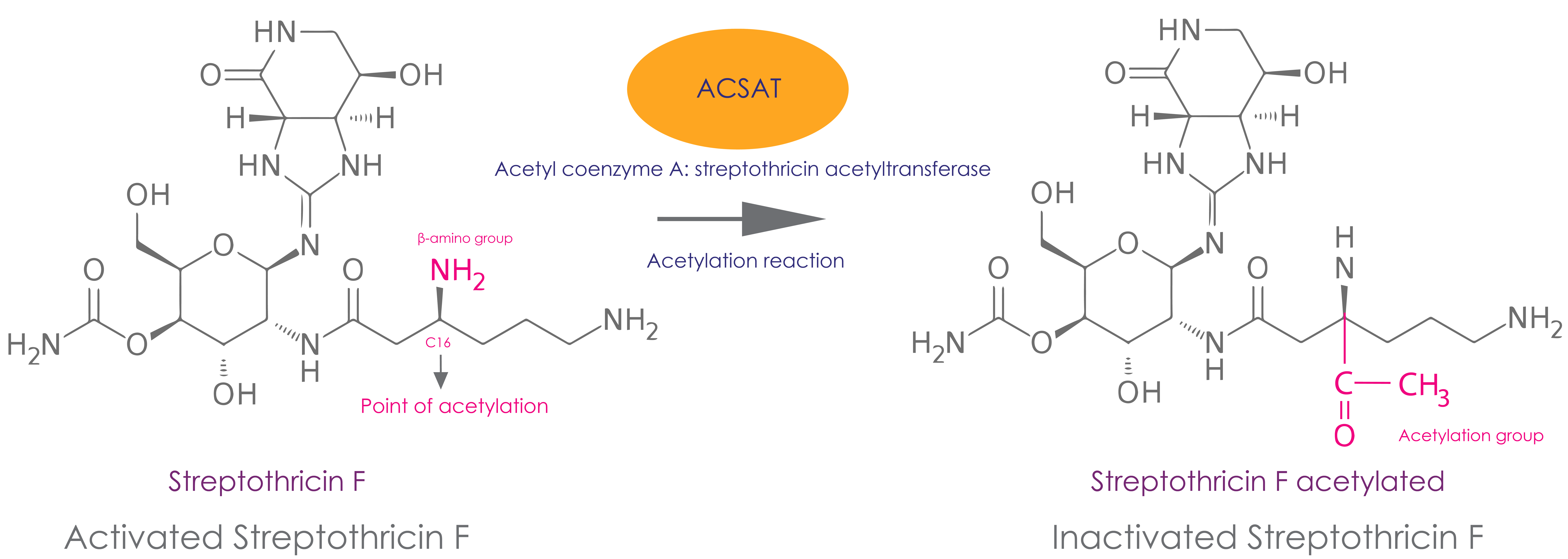
Nourseothricin is commonly used for bacterial and plant selection. It is not used in animal or human experiments because of its toxicity to kidneys (nephrotoxicity).
The concentration of nourseothricin usually ranges between 50 µg/ml to 100 µg/ml; however, concentration really depends on the organism. Tested concentrations of nourseothricin are presented in table 1.
Table 1. Concentrations of nourseothricin for different organisms.
|
Organism |
|
Species |
MIC* (µg/ml) |
Selection Concentration (µg/ml) |
|
Gram-negative bacteria |
|
Agrobacterium tumefaciens
|
|
100 |
|
Gram-negative bacteria |
|
Escherichia coli
|
2 –12 |
50 |
|
Gram-negative bacteria |
|
Francisella tularensis
|
|
50 |
|
Gram-negative bacteria |
|
Pseudomonas aeruginosa
|
50 |
100 |
|
|
|
|
|
|
|
Gram-positive bacteria |
|
Bacillus subtilis
|
5 |
50 |
|
Gram-positive bacteria |
|
Enterococcus faecium
|
8 – 256 |
500 |
|
Gram-positive bacteria |
|
Staphylococcus aureus
|
2 –12 |
50 |
|
|
|
|
|
|
|
Streptomycetes |
|
Streptomyces lividans
|
6 |
100 |
|
|
|
|
|
|
|
Yeast |
|
Candida albicans
|
200 |
250 – 450 |
|
Yeast |
|
Hansenula polymorpha
|
|
100 |
|
Yeast |
|
Kluyveromyces lactis
|
|
50 |
|
Yeast |
|
Pichia pastoris
|
|
100 |
|
Yeast |
|
Saccharomyces cerevisiae
|
25 |
75 –100 |
|
Yeast |
|
Schizosaccharomyces pombe
|
40 |
100 |
|
|
|
|
|
|
|
Other Ascomycota |
|
Acremonium chrysogenum
|
|
25 |
|
Other Ascomycota |
|
Aspergillus nidulans
|
|
120 |
|
Other Ascomycota |
|
Cryphonectria parasitica
|
|
100 |
|
Other Ascomycota |
|
Neurospora crassa
|
|
200 |
|
Other Ascomycota |
|
Penicillium chrysogenum
|
|
150 –200 |
|
Other Ascomycota |
|
Podospora anserina
|
|
50 |
|
Other Ascomycota |
|
Sordaria macrospora
|
|
50 |
|
Other Ascomycota |
|
Trichophyton mentagrophytes
|
|
50 |
|
|
|
|
|
|
|
Basidiomycota |
|
Cryptococcus neoformans
|
|
100 |
|
Basidiomycota |
|
Schizophyllum commune
|
3 |
8 |
|
Basidiomycota |
|
Ustilago maydis
|
|
75 –100 |
|
|
|
|
|
|
|
Protozoa |
|
Leishmania tarentolae, major, etc.
|
|
100 |
|
Protozoa |
|
Phytomonas serpens
|
|
100 |
|
Protozoa |
|
Plasmodium falciparum
|
75** |
|
|
Protozoa |
|
Toxoplasma gondii
|
|
500 |
|
|
|
|
|
|
|
Microalgae |
|
Phaeodactylum tricornutum
|
|
50 –250 |
|
Microalgae |
|
Thalassiosira pseudonana
|
|
100 |
|
|
|
|
|
|
|
Plants |
|
Arabidopsis thaliana
|
20 |
50 –200 |
|
Plants |
|
Daucus carota
|
|
100 |
|
Plants |
|
Lotus corniculatus
|
|
50 |
|
Plants |
|
Nicotiana tabacum
|
|
100 |
|
Plants |
|
Oryza sativa
|
20 |
200 |
Cundliffe, E., 1989. How antibiotic-producing organisms avoid suicide. Annu. Rev. Microbiol 207–233.
Derbise, A., Dyke, K.G.H., El Solh, N., 1996. Characterization of a Staphylococcus aureus transposon, Tn5405, located within Tn5404 and carrying the aminoglycoside resistance genes, aphA-3 and aadE. Plasmid 35, 174–188. https://doi.org/10.1006/plas.1996.0020
Dowgiallo, M.G., Miller, B.C., Kassu, M., Smith, K.P., Fetigan, A.D., Guo, J.J., Kirby, J.E., Manetsch, R., 2022. The convergent total synthesis and antibacterial profile of the natural product streptothricin F. Chem. Sci. 13, 3447–3453. https://doi.org/10.1039/d1sc06445b
GoldBio, 2019. Nourseothricin Sulfate, Nourseothricin sulfate.
Hahn, F., 1983. Modes and mechanisms of microbial growth inhibitors.
Haupt, I., Hübener, R., Thrum, H., 1978. Streptothricin F, an Inhibitor of Protein Synthesis with Miscoding Activity. J. Antibiot. (Tokyo). 31, 1137–1142. https://doi.org/10.7164/antibiotics.31.1137
Inamori, Y., Kato, Y., Morimoto, K., Morisaka, K., Saito, G., Sawada, Y., Taniyama, H., 1979. Toxicological approaches to streptothricin antibiotics. III. Biological studies on delayed toxicity of streptothricin antibiotics in rats. Chem. Pharm. Bull. 2091.
Makeyev, A. V., Liebhaber, S.A., 2002. The poly(C)-binding proteins: A multiplicity of functions and a search for mechanisms. Rna 8, 265–278. https://doi.org/10.1017/S1355838202024627
Robinson, H., Graessle, O., Smith, D. 1944. Studies on the Toxicity and Activity of Streptothricin. Science, New Series, Vol. 99, No. 2583
Schwabacher, H., Hughes, W.H., 1954. Bacterial Resistance to Antiseptics. Br. Med. J. 2, 247. https://doi.org/10.1136/bmj.2.4881.247
Smith, K.P., Kang, Y.-S., Green, A.B., Dowgiallo, M.G., Miller, B.C., Chiaraviglio, L., Truelson, K.A., Zulauf, K.E., Rodriguez, S., Manetsch, R., Kirby, J.E., 2021. Profiling the in vitro and in vivo activity of streptothricin-F against carbapenem-resistant Enterobacterales: a historic scaffold with a novel mechanism of action. bioRxiv doi: 10.1101/2021.06.14.448463.
Waksman, S.A., Woodruff, H.B., 1942. Streptothricin, a New Selective Bacteriostatic and Bactericidal Agent, Particularly Active Against Gram-Negative Bacteria. Proc. Soc. Exp. Biol. Med. 49, 207–210. https://doi.org/10.3181/00379727-49-13515
Wendlandt, S., Feßler, A.T., Monecke, S., Ehricht, R., Schwarz, S., Kadlec, K., 2013. The diversity of antimicrobial resistance genes among staphylococci of animal origin. Int. J. Med. Microbiol. 303, 338–349. https://doi.org/10.1016/j.ijmm.2013.02.006
Zähringer, U., Voigt, W., Seltmann, G., 1993. Noureseothricin (streptothricin) inactivated by a plasmid pIE636 encoded acetyl transferase of Escherichia coli: Location of the acetyl group. FEMS Microbiol. Lett. 110, 331–334. https://doi.org/10.1111/j.1574-6968.1993.tb06344.x

IPTG and auto-induction are two ways to induce protein expression in bacteria. They work similarly, but have different trade-offs in terms of convenience. While IPTG...

The final concentration of IPTG used for induction varies from 0.1 to 1.0 mM, with 0.5 or 1.0 mM most frequently used. For proteins with...

A His-tag is a stretch of 6-10 histidine amino acids in a row that is used for affinity purification, protein detection, and biochemical assays. His-tags...

Competent cells such as DH5a, DH10B, and BL21 will maintain their transformation efficiency for at least a year with proper storage. It is important to...
