Basic and Advanced Fluorescence Microscopy Techniques
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
Fluorescence microscopy spans from basic epifluorescence/widefield microscopy to advanced techniques like confocal microscopy, FRAP, FLIM, FRET, and TIRF. These cutting-edge technologies enable specimen imaging with exceptional resolution and precision.
Each fluorescence microscopy technique comes with its distinct characteristics, benefits and drawbacks.
In this article we will go into more detail about each microscopy technique, explaining the principle behind each, advantages and disadvantages, and other important information.
Widefield or epifluorescence microscopy
Key features of a widefield microscope
Types of widefield microscopes
Pros and cons of widefield microscopy
Key features of a confocal microscope
Pros and cons of confocal microscopy
What led to the recent advancements in fluorescence microscopy?
Widefield, also known as epifluorescence microscopy is the most basic type of fluorescence microscopy where the whole specimen is illuminated. In this technique, both the incident and emitted light waves are transmitted through the same lens.

Figure 1. An image of mouse ear cells. The target protein in this case was b-actin, labeled with green fluorescent protein (green), and its localization was imaged using an epifluorescence microscope. F-actin was labeled with phalloidin (red). Scale bar =1 micrometer. (Roy and Perrin. 2018).
Widefield microscopes have several key features including a white light source, a fluorescence light source, dichoic mirrors, filters and digital cameras.
Widefield microscope features list:
Gas arc lamps were the primary source of excitation light for widefield microscopy until the light-emitting diodes (LED) were developed and then used as fluorescent light sources.
LEDs offer several advantages over arc lamps, including extended lifespan, improved light output stability, and enhanced reproducibility and quantification.
Nonetheless, the familiarity and availability of the gas arc lamps like mercury-arc lamps and xenon-arc lamps make them a convenient choice for researchers, manufacturers, and users who have already optimized their setups for gas arc lamps.
Mercury arc lamps are also known to produce high-intensity light output, often surpassing the brightness of LEDs.
Despite being powerful light sources for fluorescence microscopy, gas arc lamps have a limited lifespan and need to be replaced frequently.
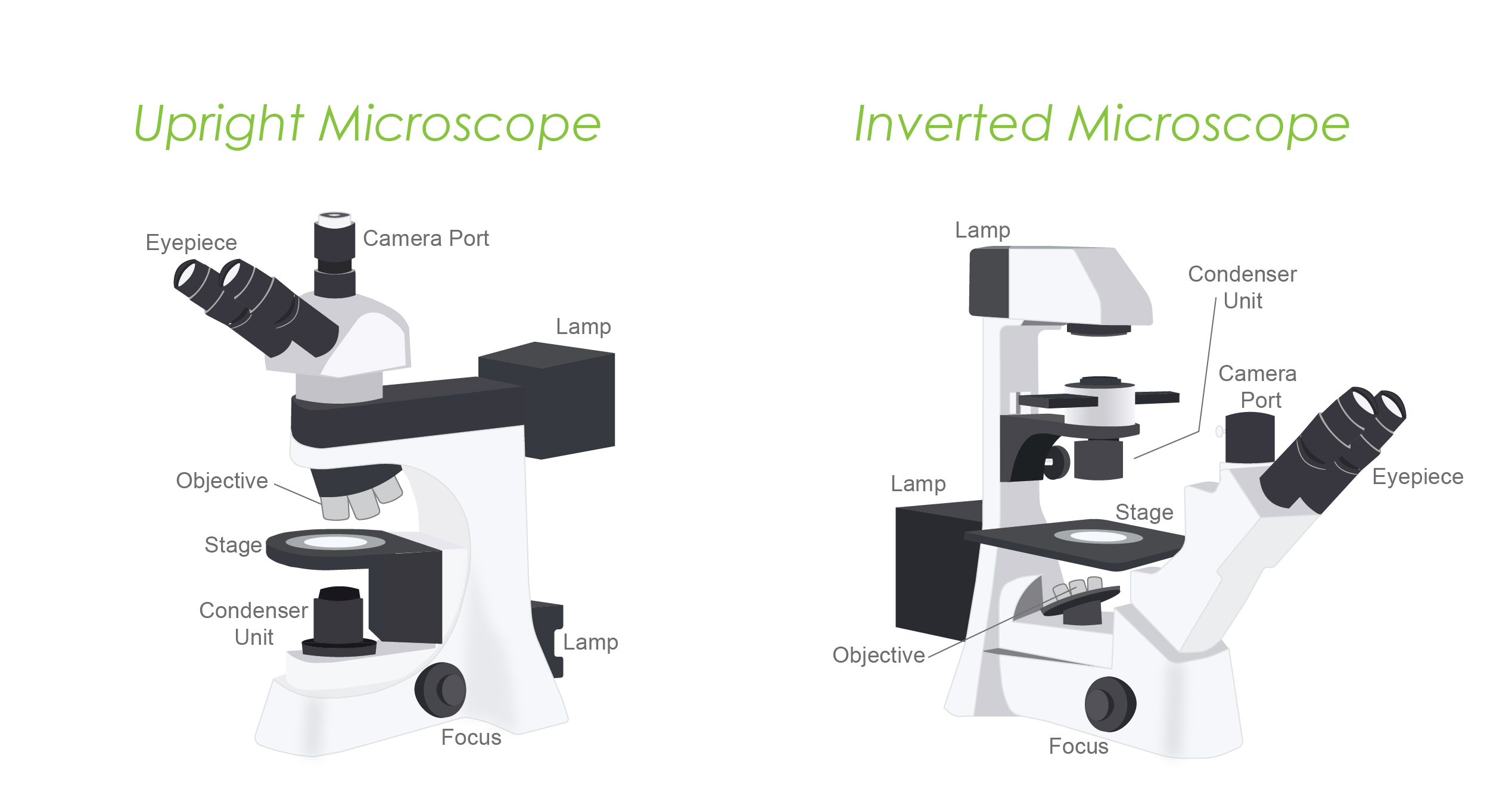
Widefield microscopes can be either inverted or upright. The distinction affects the type of specimen being studied.
Inverted microscope:
Upright microscope:
Confocal microscopy, a fluorescence optics-based technique, is used to obtain high-resolution and intricate 3D images of cellular and tissue components, including live samples. It is also useful in determining biomolecular interactions and expression patterns using co-localization studies.
Confocal microscopy was invented by Marvin Minsky in 1957 and was introduced to the commercial market in the 1980s. Since then, it has revolutionized cell and molecular biology research.
Just like widefield microscopy, in confocal microscopy, you can employ immunofluorescence to specifically label various components of living or fixed cells and tissue sections, followed by high-resolution visualization.

Figure 3. A
confocal image showing mouse ear cells inside the cochlea. The target protein
fascin-2 was labelled with GFP (green) while F-actin was labelled with
phalloidin (red).

A confocal microscope utilizes laser units integrated into the system to serve as the light source for stimulating fluorescent dyes and proteins. Because of this, it is also called confocal laser scanning microscopy (CLSM).
Like widefield microscopy, confocal microscopy utilizes fluorescent optics. However, instead of illuminating the entire specimen, confocal microscopy uses laser light to focus on a precise spot at a specific depth within the sample.
This results in fluorescence emission at that specific point, which helps image your target with high specificity.
A pinhole placed in the optical pathway acts as a filter, eliminating out-of-focus signals and enabling only the fluorescence signals from the illuminated spot to reach the detector.
This technique provides high-resolution, three-dimensional imaging with improved contrast and reduced background noise. This is what helps you get a blur-free image.
Although gas lasers are commonly used, there are newer light sources such as diode lasers, and fiber lasers that are gaining more popularity.
They generate less heat, enhance stability, uniformity, and they are able to emit wavelengths covering a wider range of the visible spectrum.
PMTs are distinct from digital cameras in that they are vacuum tubes with photons entering at one end and an electron-multiplying component present in the tube body. An electrical signal is generated by converting the collected photons prior to the image assembly and display.
Confocal systems enable image capture using both contrast and fluorescence illumination simultaneously. The images are then merged and analyzed by image analysis software built within the computer.
Two things have led to the recent advancements in fluorescence microscopy beyond widefield and confocal setups.
1) Limitations in widefield and confocal setups
2) Advancements in imaging technologies, including the increasing capabilities of computer software for image acquisition and analysis
Different microscopy techniques were subsequently developed in recent years after widefield and confocal microscopy. Advancement in one technique triggered innovation in the other.
Based on these new techniques, novel assays have also been developed.
In terms of software development, image analysis algorithms, machine learning approaches, and data visualization tools have been developed to extract quantitative information from large and complex imaging datasets. This enables researchers to obtain precise measurements and perform quantitative analyses of protein functions and processes in a high-throughput manner.
It is extremely intriguing how resourceful researchers have consistently turned technological limitations into benefits.
For instance, what may be viewed as a drawback or unwanted artifact by one researcher such as photobleaching, was ingeniously employed by another researcher.
Some of the commonly used advanced imaging techniques are discussed below.
FRAP (fluorescence recovery after photobleaching) is a fluorescence microscopy technique that enables you to study how fluorescently-labeled molecules move and behave within living samples.
Applications
FRAP is frequently employed to examine
This enables a deeper understanding of the dynamic behavior of biomolecules in their native cellular environment.
FLIM (fluorescence lifetime imaging microscopy) is an advanced and powerful technique for analyzing and visualizing the spatial distribution of specific cellular components. It provides valuable insights into the localization and dynamics of biomolecules like proteins and nucleic acids within cells.
Applications
TIRF (Total internal reflection fluorescence) is an advanced fluorescence microscopy method to visualize cellular events happening at or close to the cell membrane in living cells with a high signal-to-noise ratio.
TIRF is not ideal for visualizing structures that are located deep inside a specimen.
Applications
FRET (Fluorescence resonance energy transfer) is a robust technique that helps determine the exact location and spatial proximity of molecules labeled with fluorescent probes.
This technique not only provides valuable insights into the colocalization of molecules but also enables analysis of molecules’ associations or interactions within living cells, shedding light on cellular processes and molecular mechanisms in a non-invasive manner.
Applications
Super-resolution microscopy is a technique developed to visualize ultra-small structures within living cells or tissue that fail to be resolved by conventional widefield or confocal microscopy.
Super-resolution microscopy pushes beyond the limitations of diffraction, providing impressive 3D resolution. Using this technique, you can gain unprecedented insights into a cell’s intricate structural organization and the behavior of biomolecular assemblies at nanoscale levels.
Some examples of super-resolution microscopy include
Brown. 2007. Fluorescence microscopy-avoiding the pitfalls. J Cell Sci. 120(Pt 10):1703-5. doi: 10.1242/jcs.03433
Chen et al. 1997. Fluorescence energy transfer detection as a homogeneous DNA diagnostic method. PNAS. 94(20): 10756–10761. doi: 10.1073/pnas.94.20.10756
Elliott. 2020. Confocal Microscopy: Principles and Modern Practices. Curr protoc cytom. 92(1). doi: 10.1002/cpcy.68
Fish. 2015. Total Internal Reflection Fluorescence (TIRF) Microscopy. Curr protoc cytom. doi: 10.1002/0471142956.cy1218s50
Ishikawa-Ankerhold. 2012. Advanced Fluorescence Microscopy Techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 17(4): 4047–4132. doi: 10.3390/molecules17044047
Lippincott-Schwartz et al. 2018. The Development and Enhancement of FRAP as a Key Tool for Investigating Protein Dynamics. Biophys J. 115(7):1146-1155. doi: 10.1016/j.bpj.2018.08.007
Nakabayashi. 2017. Application of Fluorescence Lifetime Imaging (FLIM) to Measure Intracellular Environments in a Single Cell. 1035:121-133. doi: 10.1007/978-3-319-67358-5_8
Roy and Perrin. 2018. The stable actin core of mechanosensory stereocilia features continuous turnover of actin cross-linkers. MBoC. 29(15):1856-1865. doi: 10.1091/mbc.E18-03-0196
Sekar and Periasamy. 2003. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol. 160(5): 629–633. doi: 10.1083/jcb.200210140
Yang et al. 2021. Super-resolution Microscopy for Biological Imaging. Adv Exp Med Biol. 3233:23-43. doi: 10.1007/978-981-15-7627-0_2

A His-tag is a stretch of 6-10 histidine amino acids in a row that is used for affinity purification, protein detection, and biochemical assays. His-tags...

Competent cells such as DH5a, DH10B, and BL21 will maintain their transformation efficiency for at least a year with proper storage. It is important to...

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...
