An Overview of Polyclonal and Monoclonal Antibodies: Their Differences and How to Choose
by Tyasning Kroemer, Ph.D.

by Tyasning Kroemer, Ph.D.
Finding the right antibody for your protein immunoassays can be challenging. An antibody, which works well for an assay, may fail to work in another assay. Therefore, this article will provide you with helpful information about the differences between two widely used antibodies: monoclonal and polyclonal antibodies. In addition, we include some considerations before you choose an antibody.
In this article
What are monoclonal and polyclonal antibodies?
What is the difference between monoclonal antibodies and polyclonal antibodies?
How are monoclonal and polyclonal antibodies produced?
What are monoclonal and polyclonal antibodies used for?

In protein research, there are two popular types of antibodies: monoclonal antibodies and polyclonal antibodies.
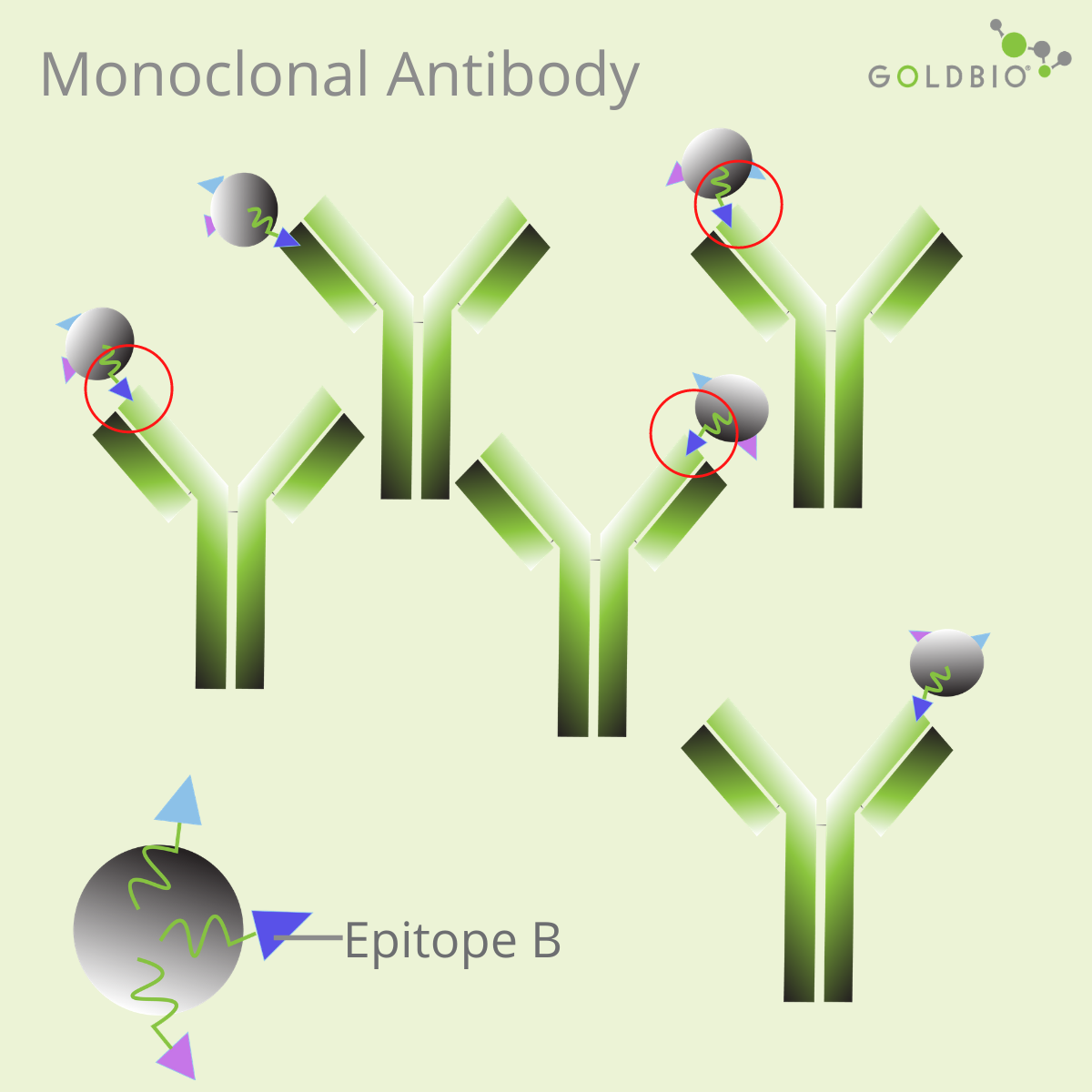

The main differences between these two types of antibodies:
The production of monoclonal antibodies starts by injecting an animal with an antigen. After several weeks, the spleen cells are harvested and fused with myeloma cells. Spleen cells produce antibodies, but they are unable to grow in culture. On the contrary, myeloma cells growing in culture are unable to produce antibody. After the fusion of both cells, the hybrid cells, also known as hybridoma cells, are selected and cultured to produce identical monoclonal antibodies.

Polyclonal antibodies are produced by injecting a particular antigen into an animal. After several days, the animal receives a booster of antigen injection to produce large amounts of antibodies against the antigen. Then, blood serum containing polyclonal antibodies are collected or purified.

Advantages:
Disadvantages
Advantages:
Disadvantages:
Monoclonal antibodies have high specificity, so they are useful for some research applications requiring antibodies specific to a single epitope. Due to this reason, producing therapeutic antibodies is one attractive approach for drug development (Lu et al., 2020).
Monoclonal antibodies are also useful for quantifying the levels of a particular target protein; for example, in ELISA or radioimmunoassay.
.png)
They can also be used for Western Blotting (WB), immunohistochemistry (IHC), and immunofluorescence assay (IF). However, depending on the epitope, monoclonal antibodies can also be ineffective for these methods. A change in the structure of protein caused by chemicals, pH, or temperature, can affect the efficiency of the monoclonal antibodies.
Monoclonal antibodies produced against native proteins may recognize a conformational epitope, or an epitope containing discontinuous amino acid residues (Liang, 1998). As a result, these monoclonal antibodies may fail to recognize the epitope on the denatured state of the target proteins.

Discontinuous residues are amino acids far apart in primary sequences, but close to each other in the folded structures. In this case, choose a monoclonal antibody that can recognize continuous residues, or linear epitopes. Otherwise, consider to use a polyclonal antibody.
Polyclonal antibodies are useful for many general research applications and diagnostic assays, because of their high affinity towards the target antigen and their tolerance to minor changes of the target protein,

Applications for polyclonal antibodies include one or more of these following methods: Western Blotting, ELISA, immunohistochemistry, immunofluorescence assay, and immunoprecipitation (IP).
If produced against a denatured antigen, polyclonal antibodies recognize epitopes of the target protein in the denatured state. Treatments during assays, such as formalin fixation during immunohistochemistry, can change the molecules of the tissue to form crosslinks and mask the epitopes (Dapson, 2009). Therefore, a polyclonal antibody, which works well on Western Blots, may fail to recognize a target protein in immunohistochemistry.
Polyclonal antibodies can cause cross-reactivity and high background noise. Therefore, when performing your assays, it is essential to include good controls, such as a purified recombinant protein in Western Blots, as a reference.
Before choosing, match the antibody with the assay and investigate the details about it, including the specificity of an antibody.
One way to validate the antibody specificity is by using knockout (KO) validation. A specific antibody validated with this method usually produces no signal on the Western Blots for the knockout cell line, but yields a good signal with the expected size for the wild-type cell line.
Antibody. (2019). Genome.gov. https://www.genome.gov/genetics-glossary/Antibody.
Antibody Approaches. (n.d.). Www-Users.med.cornell.edu. Retrieved March 1, 2021, from http://www-users.med.cornell.edu/~jawagne/Antibody_Approaches.html.
Dapson, R. (2007). Macromolecular changes caused by formalin fixation and antigen retrieval. Biotechnic & Histochemistry, 82(3), 133–140. https://doi.org/10.1080/10520290701567916.
Deng, X., Storz, U., & Doranz, B. J. (2017). Enhancing antibody patent protection using epitope mapping information. MAbs, 10(2), 204–209. https://doi.org/10.1080/19420862.2017.1402998.
Eubel, H., Braun, H.-P., & Millar, A. H. (2005). Plant Methods, 1(1), 11. https://doi.org/10.1186/1746-4811-1-11.
Ivell, R., Teerds, K., & Hoffman, G. E. (2014). Proper Application of Antibodies for Immunohistochemical Detection: Antibody Crimes and How to Prevent Them. Endocrinology, 155(3), 676–687. https://doi.org/10.1210/en.2013-1971.
Kaliyappan, K., Palanisamy, M., Duraiyan, J., & Govindarajan, R. (2012). Applications of immunohistochemistry. Journal of Pharmacy and Bioallied Sciences, 4(6), 307. https://doi.org/10.4103/0975-7406.100281.
Liang, T. Chyau. (1998). Epitopes. Encyclopedia of Immunology, 825–827. https://doi.org/10.1006/rwei.1999.0219.
Lipman, N. S., Jackson, L. R., Trudel, L. J., & Weis-Garcia, F. (2005). Monoclonal Versus Polyclonal Antibodies: Distinguishing Characteristics, Applications, and Information Resources. ILAR Journal, 46(3), 258–268. https://doi.org/10.1093/ilar.46.3.258.
Lu, R.-M., Hwang, Y.-C., Liu, I-Ju., Lee, C.-C., Tsai, H.-Z., Li, H.-J., & Wu, H.-C. (2020). Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science, 27(1). https://doi.org/10.1186/s12929-019-0592-z.
Monoclonal antibodies. (n.d.). University of Nevada, Reno School of Medicine. https://med.unr.edu/ddl/technology/monoclonal-antibodies.
Pillai-Kastoori, L., Heaton, S., Shiflett, S. D., Roberts, A. C., Solache, A., & Schutz-Geschwender, A. R. (2019). Antibody validation for Western blot: By the user, for the user. Journal of Biological Chemistry, 295(4), 926–939. https://doi.org/10.1074/jbc.ra119.010472.
Quintero-Ronderos, P., María-Teresa Arango, Castiblanco, J., Correa, N. E., & Montoya-Ortíz, G. (2013, July 18). Analysis of proteins and antibodies. Nih.gov; El Rosario University Press. https://www.ncbi.nlm.nih.gov/books/NBK459443
Scalia, C. R., Boi, G., Bolognesi, M. M., Riva, L., Manzoni, M., DeSmedt, L., Bosisio, F. M., Ronchi, S., Leone, B. E., & Cattoretti, G. (2016). Antigen Masking During Fixation and Embedding, Dissected. Journal of Histochemistry & Cytochemistry, 65(1), 5–20. https://doi.org/10.1369/0022155416673995.
Stills, H. F. (2012, January 1). Chapter 11 - Polyclonal Antibody Production (M. A. Suckow, K. A. Stevens, & R. P. Wilson, Eds.). ScienceDirect; Academic Press. https://www.sciencedirect.com/science/article/pii/B9780123809209000110.
Wittig, I., Braun, H.-P., & Schägger, H. (2006). Blue native PAGE. Nature Protocols, 1(1), 418–428. https://doi.org/10.1038/nprot.2006.62.

Competent cells such as DH5a, DH10B, and BL21 will maintain their transformation efficiency for at least a year with proper storage. It is important to...

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...
