Deep Dive Into Plant Transformation: Protoplast-Mediated, Biolistic-Mediated and Agrobacterium-Mediated Gene Transfer
by Adriana Gallego, Ph.D.

by Adriana Gallego, Ph.D.

Plant transformation is the process in which a foreign gene is introduced into a plant's genome. The methods researchers use for plant transformation include protoplast-mediated transformation, biolistic-mediated transformation and Agrobacterium-mediated transformation.
Plant genetic transformation is a powerful research tool for gene discovery and function to investigate genetically controlled traits of interest in agriculture and favor breeding programs by producing novel and genetically diverse plant materials.
Protoplast-mediated transformation involves direct uptake of DNA by naked plant cells (plants without a cell wall, or protoplasts). This process provides an expression system for researchers to identify novel candidate genes.
Usually, protoplast-mediated transformation (PMT) is reported for transient expression (genes temporarily expressed - not integrated in the genome), providing an efficient way to study many genes in a short time. In this method, protoplasts are first produced through enzymatic treatments to break the cell wall. Furthermore, staining solutions like calcofluor- white and fluorescein diacetate are used to visualize the cell membrane integrity and the cell viability respectively. Then, the direct delivery of DNA to individual plant cells is performed using polyethylene glycol (PEG) or electroporation.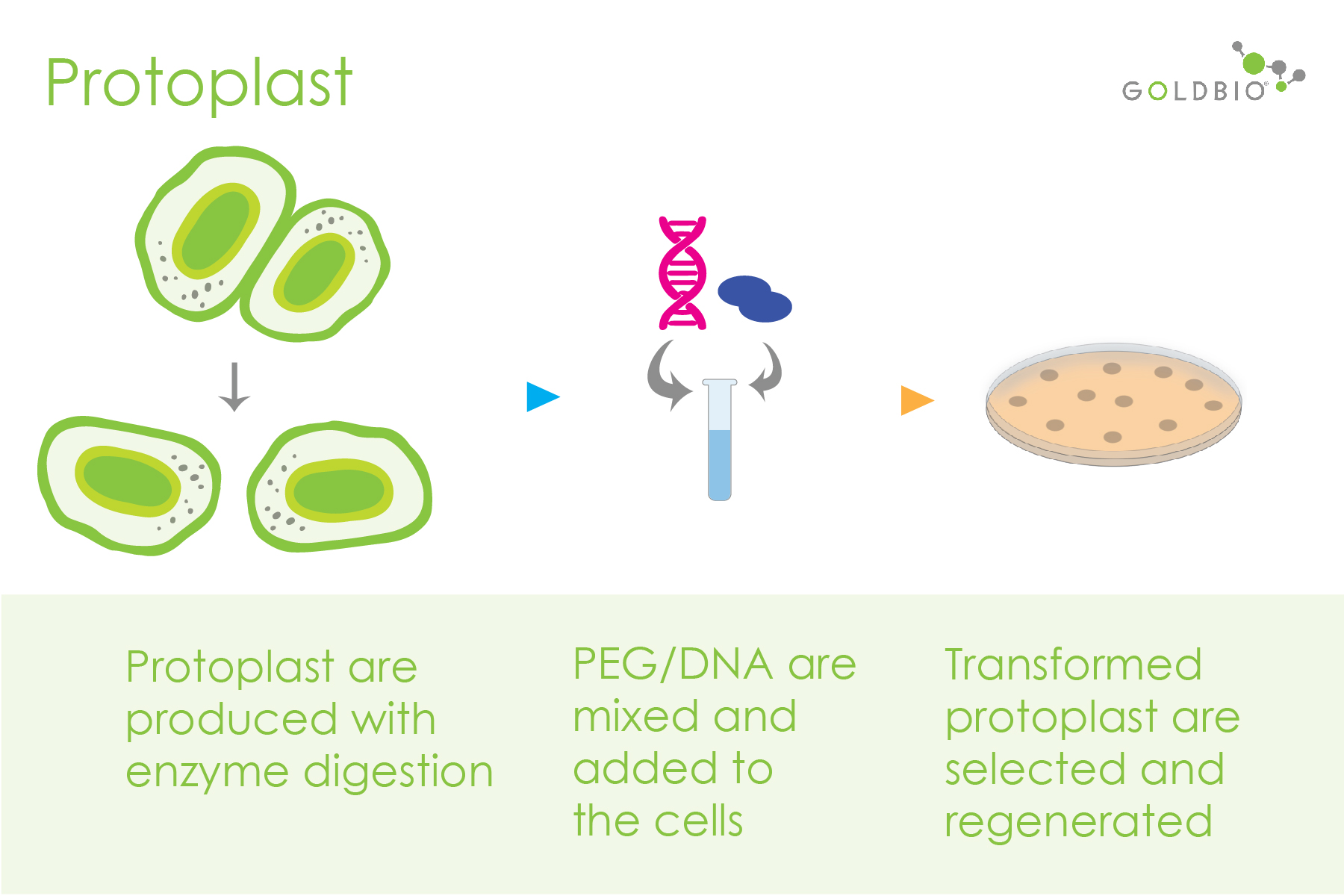
Figure 1. Protoplast-mediated transformation steps.
Finally, transformed protoplasts are selectively cultivated and used for further regeneration steps. An updated step by step protoplast transformation protocol can be found here.
Biolistic transformation, also known as particle-mediated gene transfer, was first reported in 1987 as an alternative to PMT, especially for recalcitrant specie.
Biolistic transformation can be defined as the introduction of substances into intact cells and tissues through high-velocity microprojectiles. The term came from cells shot with DNA. Since the biolistic process is a direct gene transfer method like PMT, it has no immediate effect on the DNA's chromosomal integration mechanism. Thus, the integration event arising from the biolistic process will depend upon the DNA being delivered and the organism's biology to transform.
into a gene gun, and pressurized gas like helium, provides the force. Then, some of the metal particles will penetrate the cell membranes and deliver DNA constructs ito cells. A protocol for plants using biolistics can be found here.
In this method, the natural ability of the soil bacterium Agrobacterium tumefaciens to transform host plants is exploited to develop transgenic plants. An excellent video to summarize the in vivo process can be found here.
Agrobacterium naturally transfers DNA (T-DNA) located on the tumor-inducing (Ti) plasmid into the nucleus of plant cells and stably incorporates the DNA into the plant genome. Thus, Agrobacterium-mediated gene transfer (AMGT) is widely used for stable plant transformation.
The Agrobacterium-mediated transformation process involves the following:
When it comes to producing transgenic plants, Agrobacterium-mediated transformation is a very popular method to use for several reasons:
A recent advantage of Agrobacterium-mediated gene transfer comes with the advent of gene-editing tools like CRISPR/Cas.
Genome editing technologies provide powerful tools for precise manipulation of targeted genome sequences, setting up unprecedented opportunities for crop breeding and functional genomics research. However, the lack of appropriate methods to deliver genome-editing reagents (for example, constructs encoding the nuclease and target-specific RNAs) is the primary barrier to CRISPR/Cas-mediated gene editing in a variety of plants.
Currently, Agrobacterium-mediated transformation is the preferred method of CRISPR/Cas reagent delivery. Studies have already reported this success in maize, wheat and tobacco. Also, compared to the biolistics approach, the Agro-infiltration systems have significantly improved, progressing from the single Ti plasmid to the binary vector. Even today, more advanced Agrobacterium vectors have been developed like the superbinary and ternary vectors systems (Zhang et al., 2020).
In a binary vector, T-DNA could be separated from the Ti plasmid and placed on shuttle vectors. In the superbinary vector, the binary vector carries additional virulence genes and enhances Agrobacterium-mediated gene transfer of recalcitrant plants.
With a ternary vector, a third plasmid—called the accessory plasmid or the virulence helper plasmid—is used to carry the virulence gene cluster. These improvements have increased transformation efficiency throughout Agrobacterium and expanded the transformability of a wide range of plant genotypes!

Carter, M., & Shieh, J. (2015). Gene Delivery Strategies. In Guide to Research Techniques in Neuroscience (pp. 239-252). https://doi.org/10.1016/b978-0-12-800511-8.00011-3
Duarte, P., Ribeiro, D., Carqueijeiro, I., Bettencourt, S., & Sottomayor, M. (2016). Protoplast Transformation as a Plant-Transferable Transient Expression System. Methods Mol Biol, 1405, 137-148. https://doi.org/10.1007/978-1-4939-3393-8_13
Fu, Q., Li, C., Tang, M., Tao, Y. B., Pan, B. Z., Zhang, L., Niu, L., He, H., Wang, X., & Xu, Z. F. (2015). An efficient protocol for Agrobacterium-mediated transformation of the biofuel plant Jatropha curcas by optimizing kanamycin concentration and duration of delayed selection. Plant Biotechnol Rep, 9(6), 405-416. https://doi.org/10.1007/s11816-015-0377-0
Heenatigala, P. P. M., Yang, J., Bishopp, A., Sun, Z., Li, G., Kumar, S., Hu, S., Wu, Z., Lin, W., Yao, L., Duan, P., & Hou, H. (2018). Development of Efficient Protocols for Stable and Transient Gene Transformation for Wolffia Globosa Using Agrobacterium. Front Chem, 6, 227. https://doi.org/10.3389/fchem.2018.00227
Ismagul, A., Yang, N., Maltseva, E., Iskakova, G., Mazonka, I., Skiba, Y., Bi, H., Eliby, S., Jatayev, S., Shavrukov, Y., Borisjuk, N., & Langridge, P. (2018). A biolistic method for high-throughput production of transgenic wheat plants with single gene insertions. BMC Plant Biol, 18(1), 135. https://doi.org/10.1186/s12870-018-1326-1
Jelly, N. S., Valat, L., Walter, B., & Maillot, P. (2014). Transient expression assays in grapevine: a step towards genetic improvement. Plant Biotechnol J, 12(9), 1231-1245. https://doi.org/10.1111/pbi.12294
Jenes, B., Moore, H.; Cao, J.; Zhang, W.; Wu, R. Techniques for gene transfer. In: Kung, S.; Wu, R. (Ed.) Transgenic plants. New York: Academic Press, 1993. cap.4, p.125-146.
Jones, H. D., Doherty, A., & Wu, H. (2005). Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods, 1(1), 5. https://doi.org/10.1186/1746-4811-1-5
Keshavareddy, G., Kumar, A. R. V., & S. Ramu, V. (2018). Methods of Plant Transformation- A Review. International Journal of Current Microbiology and Applied Sciences, 7(07), 2656-2668. https://doi.org/10.20546/ijcmas.2018.707.312
Kikkert, J., Vidal, J., & Reisch, B. (2005). Stable Transformation of Plant Cells by Particle Bombardment/Biolistics. In L. Peña (Ed.), Methods in Molecular Biology, Transgenic Plants: Methods and Protocols (Vol. 286). Humana Press Inc.
Kopbayev, A. (2004). Biolistic and Agrobacterium-mediated genetic transformation of immature and mature embryos of spring wheat cultivar SARATOVSKAYA-29 Texas A&M University].
Lardon, R., & Geelen, D. (2020). Natural Variation in Plant Pluripotency and Regeneration. Plants (Basel), 9(10). https://doi.org/10.3390/plants9101261
Lee, K., Zhu, H., Yang, B., & Wang, K. (2019). An Agrobacterium-mediated CRISPR/Cas9 Platform for Genome Editing in Maize. Methods Mol Biol, 1917, 121-143. https://doi.org/10.1007/978-1-4939-8991-1_10
Martins, P. K., Ribeiro, A. P., Cunha, B., Kobayashi, A. K., & Molinari, H. B. C. (2015). A simple and highly efficient Agrobacterium-mediated transformation protocol for Setaria viridis. Biotechnol Rep (Amst), 6, 41-44. https://doi.org/10.1016/j.btre.2015.02.002
Mathur, J., & Koncz, C. (1998). PEG-Mediated Protoplast Transformation with Naked DNA. In J. Martinez-Zapater & J. Salinas (Eds.), Methods in Molecular Biology: Arabidopsis Protocols (Vol. 82). Humana Press Inc.
Sanford, J. (1990). Biolistic plant transformation. Physiologia Plantarum, 79, 206-209.
Schmitz, D. J., Ali, Z., Wang, C., Aljedaani, F., Hooykaas, P. J. J., Mahfouz, M., & de Pater, S. (2020). CRISPR/Cas9 Mutagenesis by Translocation of Cas9 Protein Into Plant Cells via the Agrobacterium Type IV Secretion System. Frontiers in Genome Editing, 2. https://doi.org/10.3389/fgeed.2020.00006
Shillito, R. (1999). Methods of Genetic Transformation: Electroporation and Polyethylene Glycol Treatment. In Molecular improvement of cereal crops. Springer, Dordrecht. https://doi.org/https://doi.org/10.1007/978-94-011...
Song, G. Q., Prieto, H., & Orbovic, V. (2019). Agrobacterium-Mediated Transformation of Tree Fruit Crops: Methods, Progress, and Challenges. Front Plant Sci, 10, 226. https://doi.org/10.3389/fpls.2019.00226
Weyda, I., Yang, L., Vang, J., Ahring, B. K., Lubeck, M., & Lubeck, P. S. (2017). A comparison of Agrobacterium-mediated transformation and protoplast-mediated transformation with CRISPR-Cas9 and bipartite gene targeting substrates, as effective gene targeting tools for Aspergillus carbonarius. J Microbiol Methods, 135, 26-34. https://doi.org/10.1016/j.mimet.2017.01.015
Zhang, Y., Chen, M., Siemiatkowska, B., Toleco, M. R., Jing, Y., Strotmann, V., Zhang, J., Stahl, Y., & Fernie, A. R. (2020). A Highly Efficient Agrobacterium-mediated Method for Transient Gene Expression and Functional Studies in Multiple Plant Species. Plant Commun, 1(5), 100028. https://doi.org/10.1016/j.xplc.2020.100028
Zhang, Y., Zhang, Q., & Chen, Q. J. (2020). Agrobacterium-mediated delivery of CRISPR/Cas reagents for genome editing in plants enters an era of ternary vector systems. Sci China Life Sci, 63(10), 1491-1498. https://doi.org/10.1007/s11427-020-1685-9
Zhang, Z., Hua, L., Gupta, A., Tricoli, D., Edwards, K. J., Yang, B., & Li, W. (2019). Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol J, 17(8), 1623-1635. https://doi.org/10.1111/pbi.13088
Zhang, z., Li, X., Ma, S., Shan, N., Zhang, X., Sui, X., & zhang, z. (2017). A Protocol for Agrobacterium-mediated Transformation of Cucumber (Cucumis sativus L.) from cotyledon explants. Protocol Exchange. https://doi.org/10.1038/protex.2017.107

A His-tag is a stretch of 6-10 histidine amino acids in a row that is used for affinity purification, protein detection, and biochemical assays. His-tags...

Competent cells such as DH5a, DH10B, and BL21 will maintain their transformation efficiency for at least a year with proper storage. It is important to...

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...
