Parkinson’s disease is a neurodegenerative disorder, leading to tremors or trembling, stiffness, and many problems with movements (such as walking, balance, and coordination). Although not considered a deadly disease, people with Parkinson’s disease are at high risk of two major causes of death: pneumonia and falling.
In the U.S., Parkinson’s affects nearly 1 million people and more than 6 million people worldwide. Unfortunately, similar to other neurodegenerative disorders, there have been no disease-curing treatment options for Parkinson’s disease.
However, a new hope to finding an effective cure for this disease has risen, after a research team at University of California San Diego accidentally reprogrammed connective tissue cells (fibroblasts) into neurons by making a particular protein go away.
After this breakthrough, the team took a huge step forward and performed a study in mice to develop a unique approach to treat Parkinson’s disease—by generating new neurons in the damaged brain and rejuvenating the loss of neurons.
Neurodegeneration in Parkinson’s Disease
Numerous problems with movements in Parkinson’s disease result from neurodegeneration (when neurons stop working or die) in the brain, mainly from the loss of neurons.
Impaired Dopaminergic Neurons
Dopamine-producing neurons or dopaminergic neurons become impaired and die in the midbrain (substantia nigra region) of people with Parkinson’s disease. When dopaminergic neurons are dead, this condition disrupts the communication between neurons, leading to many movement problems. As Parkinson’s disease progresses to more advanced stages, more neurons die off.

Toxic Alpha-synuclein Protein Aggregations
Abnormal and toxic clumps of a misfolded alpha-synuclein protein develop inside neurons of patients with Parkinson’s disease, usually located at the tip of neurons or a synapse. These toxic clumps affect the release of dopamine between neurons, critical to control the start and stop of movements. Eventually, these clumps kill neurons.
Loss of Norepinephrine Neurons
Not only dopaminergic neurons, but patients with Parkinson’s also lose another type of neuron that produces norepinephrine. In a healthy brain, norepinephrine is responsible for supporting the proper release of dopamine and controlling many automatic functions of the body, such as heart rate and blood pressure.
Unfortunately, many current treatments are unable to treat patients with advance stages of this disease, particularly when most dopaminergic neurons are dead. Once lost, these neurons won’t recover or regenerate. Consequently, many patients at the later stages of this disease need their wheelchairs and entirely depend on their caregivers around-the-clock.
For patients with early stages of Parkinson’s disease, current treatments can help to control the symptoms, so they are comfortable to move around and able to perform their daily tasks.
Possible Treatments for Parkinson’s Disease
To ease the symptoms and help with the movement problems, some approaches for treating patients with early stages of Parkinson’s disease are:
- Making more dopamine: by converting dopamine precursors (such as levodopa drug).
- Easing some of the symptoms: by stimulating other neurotransmitters in the body (for example to stimulate acetylcholine and reduce tremors).
- Controlling the non-motor symptoms of the disease.
In addition to treating the symptoms, another possible approach to treat the patient’s brain is by targeting a pathological protein or a disease-causing protein by using antisense oligonucleotides. By doing so, this treatment will slow down or protect dopaminergic neurons from dying.

Antisense oligonucleotides. Antisense oligonucleotides are short, synthetic, single-stranded oligodeoxynucleotides (typically 16-22 bases long) which bind to mRNAs. 1) This binding recruits RNase H1 to degrade the mRNA portion of the DNA-RNA pair. 2) Since mRNA is important to code a specific protein, antisense oligonucleotides reduce the production of a pathological protein (or a disease-causing protein).
To treat Parkinson’s disease, an antisense oligonucleotide drug candidate targeting leucine-rich repeat kinase 2 (LRRK2) protein is currently under clinical trials. In a preclinical study, this treatment effectively protected neurons in mice by depleting the alpha synuclein clumps and dopaminergic neuron loss (Zhao et al. 2017).
Another preclinical study in mice by Dr. Xiang-Dong Fu’s team at UC San Diego developed a completely different approach to cure Parkinson’s disease using antisense oligonucleotides. The goal for this approach is to replace the loss of dopaminergic neurons by repopulating functional neurons in the damaged brain.
Reprograming Astrocytes into Functional Neurons using Antisense Oligonucleotide
In order to generate new neurons in the brain, researchers reprogrammed astrocytes to become functional neurons (Qian et al. 2020). Astrocytes are star-shape cells surrounding neurons that outnumber neurons by over fivefold. Unlike neurons, astrocytes are unable to fire electrical signals to communicate with each other.

Reprogramming astrocytes into neurons is only possible by reducing
the levels of polypyrimidine tract-binding protein (PTB). PTB is a protein that
binds RNA, important for turning genes on and off. This protein is also important
in reprograming a cell into a functional neuron (
Xue
et al. 2013). By forcing PTB to
disappear, the cell receives a signal to turn on the genes to produce neurons.
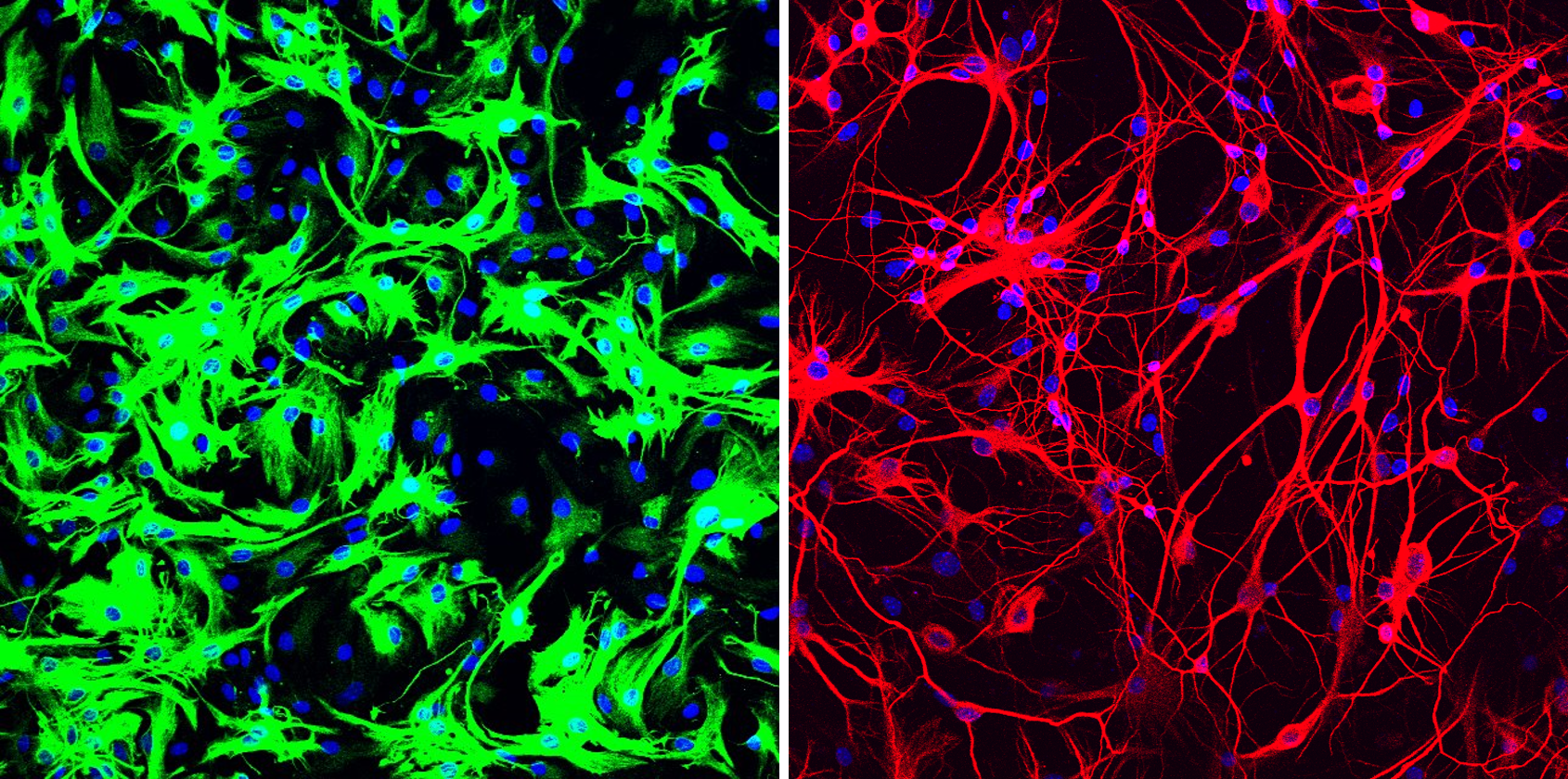
Mouse astrocytes before and after reprogramming. Left: mouse astrocytes (green) before reprogramming; Right: neurons (red) induced from mouse astrocytes after reprogramming with PTB antisense oligonucleotide treatment. [Credit: UC San Diego Health Sciences]
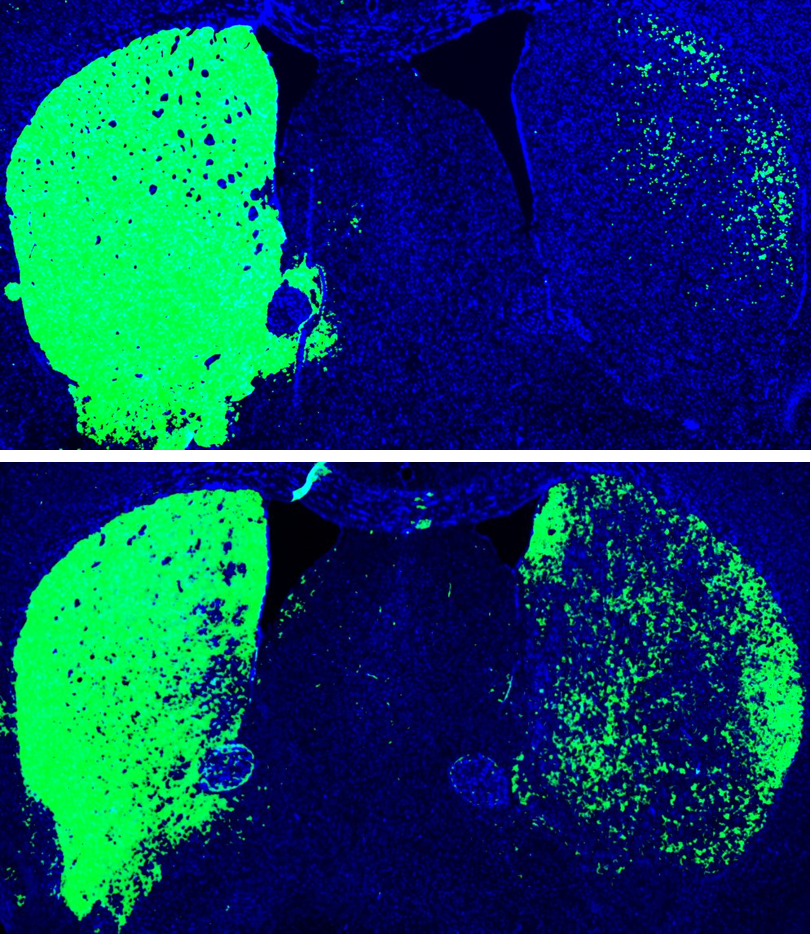
Mouse brain before and after reprogramming. Top: Mouse brain before reprogramming, with dopaminergic neurons shown (green). Bottom: Mouse brain after reprogramming with PTB antisense oligonucleotide treatment, which converted astrocytes into more dopaminergic neurons (green). [Credit: UC San Diego Health Sciences]
The team observed that a single antisense oligonucleotide treatment in those treated mice successfully reprogrammed a small number of astrocytes into functional neurons and restored a level of dopamine similar to that in normal mice.
Although this study using mice as a model is still preliminary, the idea of using antisense oligonucleotides to repopulate neurons in the brain could pave the way to find a better treatment for patients with Parkinson’s disease.
This new approach for treating Parkinson’s disease appears promising and it might help cure patients in advanced stages of this disease. What’s more exciting is this treatment could potentially be useful to treat patients with other neurodegenerative diseases, such as Alzheimer's and Huntington's diseases, by rejuvenating the loss of neurons.
References
Alpha-synuclein and Parkinson’s. (2016, March 15). The Cure Parkinson’s Trust. https://www.cureparkinsons.org.uk/why-alpha-synuclein.
Arenas, E. (2020). Method to combat Parkinson’s disease by astrocyte-to-neuron conversion. In: Nature Publishing Group.
Denworth, L. (2020). A Breakthrough in Genetic Medicine for Rare Diseases. Scientific American.
Fahn, S., & Sulzer, D. (2004). Neurodegeneration and Neuroprotection in Parkinson Disease. NeuroRx, 1(1), 139–154.
Leavitt, B. R., & Tabrizi, S. J. (2020). Antisense oligonucleotides for neurodegeneration. Science, 367(6485), 1428–1429. https://doi.org/10.1126/science.aba4624.
Parkinson’s Disease. (2017). Parkinson’s Disease. National Institute on Aging. https://www.nia.nih.gov/health/parkinsons-disease.
One-time treatment generates new neurons, eliminates Parkinson’s disease in mice. (n.d.). EurekAlert! Retrieved July 2, 2020, from https://www.eurekalert.org/pub_releases/2020-06/uo...
One-Time Treatment Generates New Neurons, Eliminates
Parkinson’s Disease in Mice. (n.d.). UC Health - UC San Diego.
https://health.ucsd.edu/news/releases/Pages/2020-0...
One-time treatment generates new neurons, eliminates Parkinson’s disease in mice: Inhibiting a single gene converts many cell types directly into dopamine-producing neurons. (n.d.). ScienceDaily. https://www.sciencedaily.com/releases/2020/06/200625102540.htm.
Qian, H., Kang, X., Hu, J., Zhang, D., Liang, Z., Meng, F., . . . Dowdy, S. F. (2020). Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature, 582(7813), 550-556.
Parkinson’s Disease: Hope Through Research | National Institute of Neurological Disorders and Stroke. (2017). Nih.Gov. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Parkinsons-Disease-Hope-Through-Research.
Rommelfanger, K., & Weinshenker, D. (2007). Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochemical pharmacology, 74(2), 177-190.
Sofroniew, M. V., & Vinters, H. V. (2009). Astrocytes: biology and pathology. Acta Neuropathologica, 119(1), 7–35. https://doi.org/10.1007/s00401-009-0619-8.
What Is Parkinson’s? (2019, January 9). Parkinson’s Foundation. https://www.parkinson.org/understanding-parkinsons/what-is-parkinsons.
Xue, Y., Ouyang, K., Huang, J., Zhou, Y., Ouyang, H., Li, H., . . . Bi, Y. (2013). Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell, 152(1-2), 82-96.
Zhao, H. T., John, N., Delic, V., Ikeda-Lee, K., Kim, A., Weihofen, A., . . . Volpicelli-Daley, L. A. (2017). LRRK2 antisense oligonucleotides ameliorate α-synuclein inclusion formation in a Parkinson’s disease mouse model. Molecular Therapy-Nucleic Acids, 8, 508-519.





