What is ion exchange chromatography?
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Have you ever heard the saying “opposites attract”? Well, it turns out this phrase is true in the molecular world as well. Positive and negative molecules attract and interact with one another.
Charge complementarity is often observed at the interface between biological molecules such as proteins, nucleic acids, and lipids. This means that a positive surface of one molecule binds to the negative surface of another molecule (Figure 1)(Mishra and Levy, 2015; Pérez-Cano & Fernández-Recio, 2010; Thakur et al, 2023; Weber and Steitz, 1984). If you want to know more about the biological relevance of protein charge, this is the perfect article for you!
A protein’s charge can also be leveraged to enable its purification.
Ion-exchange chromatography separates molecules, such as proteins, by charge. You bind your protein of interest to a column of the opposite charge. Then, by controlling the pH and ionic strength of your purification buffers you wash away contaminating proteins and finally elute your target protein.
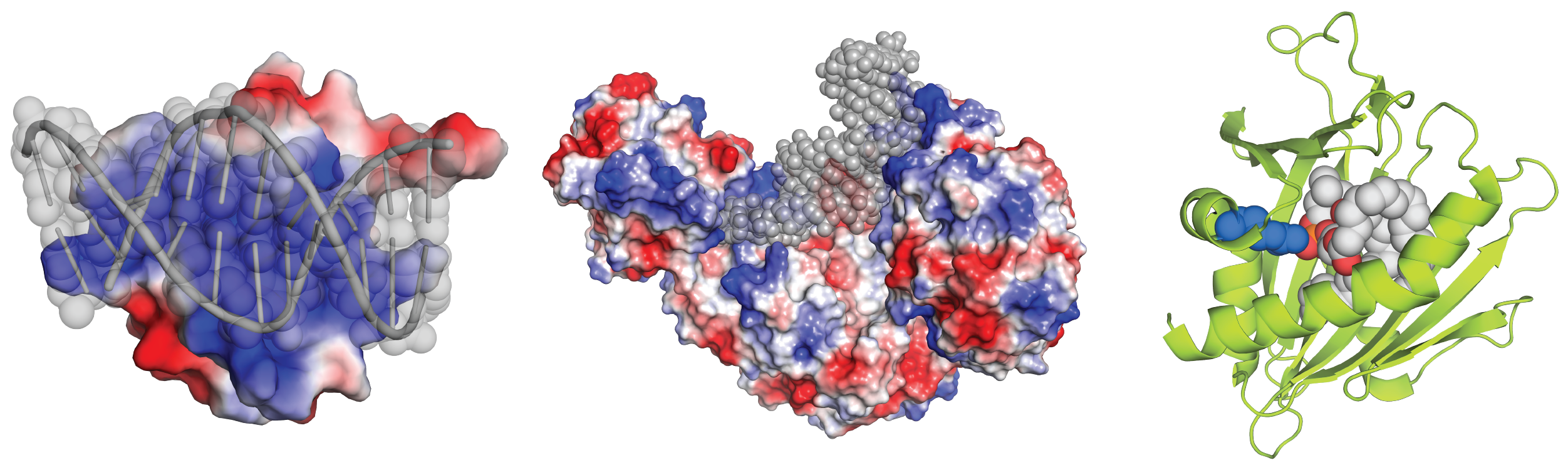
Figure 1. Protein charge is important in interactions with DNA (left), RNA (middle), and lipids (right). The DNA, RNA and lipids are colored gray. Protein is colored according to charge in left and middle images – blue is positively, and red is negatively charged. On the right image, an arginine residue (blue spheres) is important for the protein’s (green) interaction with negatively charged lipid head groups (red). Structures from PDB files 4IRI, 3SNP, and 1LN1.
In this article we’ll cover some of the basics about what ion exchange chromatography is, how to use it, and some specialized purification uses that ion exchange really excels at. Throughout the article you’ll see hyperlinks to other articles that cover related topics in more depth.
Ion Exchange Chromatography Purification Protocol
Important Uses for Ion Exchange Chromatography
There are two main types of ion exchange: cation exchange and anion exchange chromatography.
Cation exchange resins are negatively charged and bind to positively charged proteins. That’s why they’re called cation exchange, because they bind to cationic proteins.
Alternatively, anion exchange resins are positively charged and bind to negatively charged proteins. They bind to and exchange anionic proteins.
Whichever type you use, ion exchange chromatography is performed in resin columns. These can either be hand packed, like the columns at the end of this article, or you can purchase more expensive prepacked columns.
Ok, just to reiterate one more time, a cation exchange resin is negatively charged and binds positively charged proteins. Conversely, anion exchange resins are positively charged and bind negatively charged proteins (Figure 2).
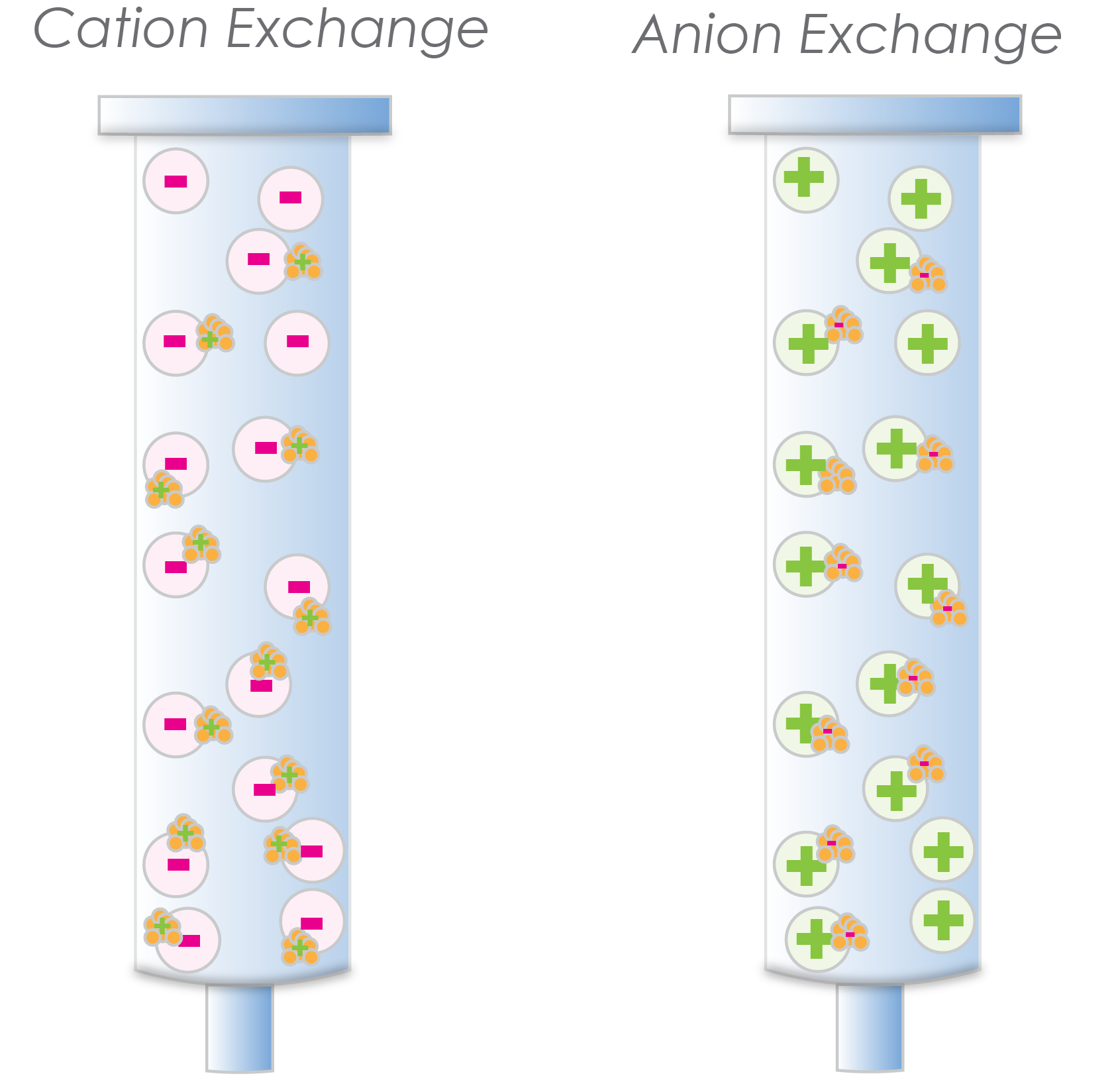
Figure 2. The cation exchange resin has negatively charged beads that bind positively charged proteins (left). The anion exchange resin has positively charged beads that bind negatively charged proteins (right).
So, it is important to know what the charge of your protein will be in the buffer that you are using to purify it. This article, discusses the relationship between protein charge and buffer pH, so check it out if you need a refresher in this area.
There are a few different types of cation exchange and anion exchange resins. In addition, ion exchange resins have different resolutions which can be important depending on why exactly you are doing this type of purification for. Check out this article for more detail on different types of cation and anion exchange resins and resin resolution.

Like affinity tag purifications, ion exchange protocols include discrete load, wash, and elute steps (Figure 3). The load step is when you load your protein sample onto the column, the wash step washes away some contaminating proteins, then the elution step is when you get your protein back off of the column.
For the load step, you will want your salt concentration to be low enough that your protein will stick to the column. If you’re using NaCl, somewhere between 0 and 150mM is typically a good concentration for loading your protein onto the column.
There are lots of other types of salts you might use in your protein purification buffer too, and we cover those in this article. If using a different type of salt, don’t consider the suggested NaCl concentration I listed above as the concentration range will be different depending on which salt you’re using, though the general concept remains the same.
For the wash step people usually use the same buffer that they loaded the column with. Sometimes you’ll want to go to a slightly higher salt concentration in this buffer, which can help wash away contaminating molecules. Just make sure the salt concentration isn’t so high that it also washes away your protein of interest!
Ok, now on the elution step you will raise the salt concentration high enough to elute your protein of interest. You can use a step elution, a gradient elution, or a combination of the two.
While this might seem simple, it can quickly get more complex, especially on the elution step when you’re deciding which type of elution to execute.
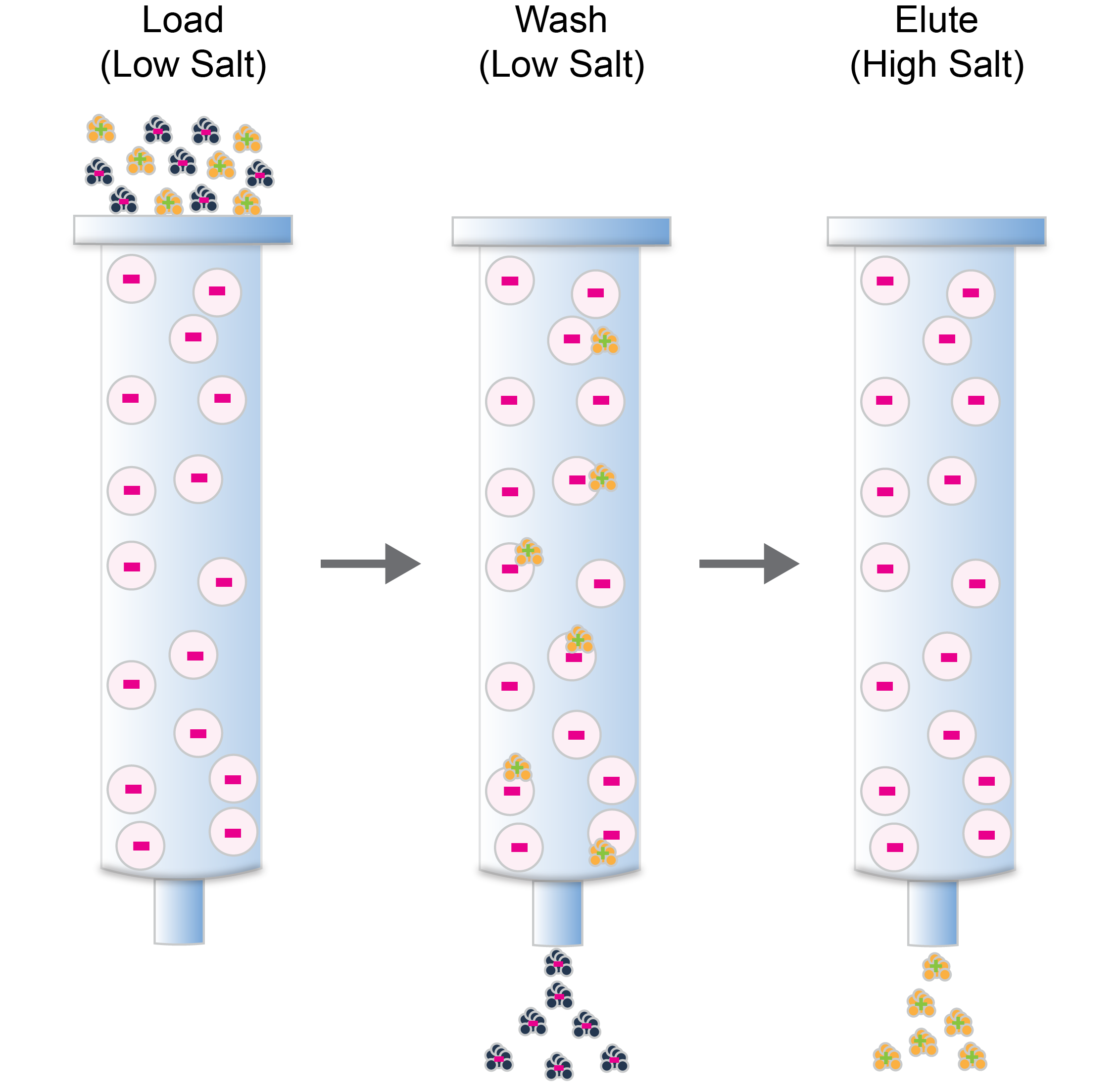
Figure 3. Ion-exchange chromatography has distinct load (left), wash (middle), and elute (right) steps.
Ion Exchange Chromatography is routinely used as a protein purification step in an overall protocol that also features affinity and size exclusion purifications. However, ion exchange also has some advantages and specific uses compared to these other purification methods, including:
We cover these specific uses of ion exchange chromatography in more detail here.
So there’s a broad overview on ion exchange chromatography. You probably noticed there are a lot of links throughout this article to related subjects, so we’ve also added them all here for easy access:

The protein streptavidin clamps onto the small molecule biotin in one of the tightest natural interactions ever discovered. Over the years, the strength of this...

Maybe you are about to start working with D-luciferin, and you begin reviewing protocols. But what you’re doing is a little different. Or what you...

Handles are common in everyday life. We use them to help us open doors, cabinets, or to hold coffee mugs, cookware, and tools. They are...

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...
