The 2 Common Types of Ion Exchange Chromatography: Anion vs. Cation
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Ion exchange chromatography includes a suite of related methods that purify proteins based on their charge.
The two main types of ion exchange resins are anion and cation, which bind to negatively and positively charged molecules, respectively. There are several functional groups for anion and cation exchange chromatography, as well as different resolutions.
Choosing the appropriate resin and resolution type will depend on your protein of interest and purification purpose.
If you know that you need to do ion exchange chromatography to purify your protein, but you’re not sure exactly what type of ion exchange you should do – you’re in the right place! Follow along as we discuss the different types of resins used for ion exchange.
Cation Exchange vs Anion Exchange
Buffer pH in Ion Exchange Chromatography
There are two main types of ion exchange: cation exchange and anion exchange chromatography. Let’s go through exactly what these are, because the nomenclature can seem counterintuitive at first.
A cation-exchange resin is actually negatively charged and binds positively-charged proteins. Conversely, anion-exchange resins are positively charged and bind negatively-charged proteins (Figure 1).
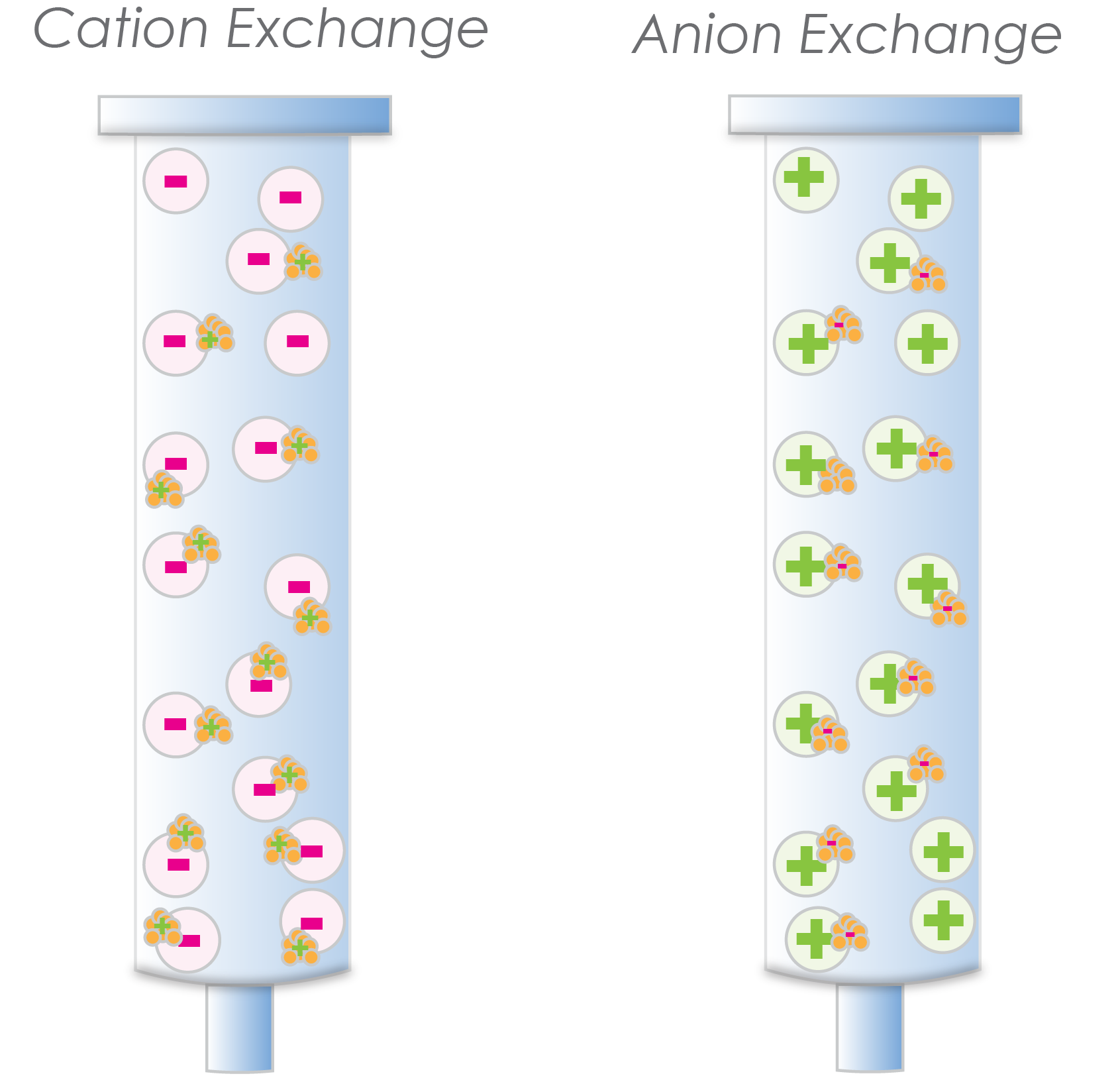
Figure 1. Cation exchange resin has negatively charged agarose beads that bind positively charged proteins (left). Anion exchange resin has positively charged agarose beads that bind negatively charged proteins (right).
So the first thing to figure out is the charge of the protein that you are trying to purify.

A protein’s charge, however, depends on the pH of the buffer or the environment that it is in. There are a lot of important features of protein purification buffers, and we discuss many of those features in depth here. For ion exchange chromatography buffers in particular, buffer pH and salt concentration are the two important variables that we will focus on in this article.
To determine whether your protein will be positive or negative at a given pH, you need to calculate the isoelectric point, conventionally denoted as “pI,” of a protein. You can check out this article to learn how to estimate a protein’s pI, and how to use that information to determine which pH will be good for purifying your protein via ion exchange.
There are many different cation exchange resins. They all bind positively charged molecules, but they use slightly different functional groups to do so. See Table 1 for a list of commonly used cation-exchange resins.
Table 1. Cation exchange functional groups.
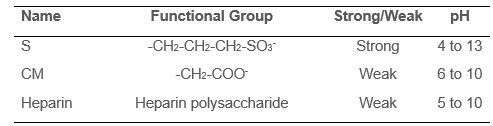
One distinction you will see in Table 1 is strong vs. weak cation exchangers. Strong indicates that a chemical moiety remains charged, and therefore useful for binding cations, across a broad pH range whereas weak exchangers are useful over a more limited pH range.
S columns are strong exchangers and are frequently used as a standard default choice for cation exchange. However, if you are working off of an existing protocol that calls for another type of cation exchange resin, you will probably want to try that type of resin first.
Heparin columns combine features of ion exchange (IEX) columns and affinity columns in that they are especially good at binding positively-charged proteins that bind to polysaccharides or nucleic acids (Siri et al, 1986), so they are a good choice for these types of proteins.
Similarly, there are many different types of anion exchange resins that bind negatively charged molecules using different functional groups. See Table 2 for a list of frequently used anion exchange resins.
Table 2. Anion exchange functional groups.
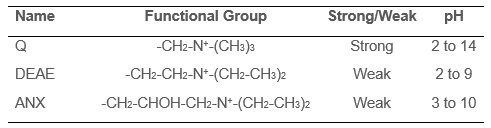
Just like cation exchangers, strong anion exchangers are charged over a broad pH range whereas weak exchangers work over a more limited pH range.
Q columns are strong anion exchangers and are frequently used as the default choice, so if you’re not sure what to use, start there. However, if you’re working off an existing protocol that uses a different anion exchange resin, you should try the same one first.
DEAE columns are particularly good at binding to nucleic acids and are frequently used to purify DNA and RNA away from a protein during a purification (Ali et al, 2017).
Whether using cation or anion exchange resins, you will also need to select whether to use a resin that is high or moderate resolution. Resolution refers to the ability of the column to separate out different proteins that have similar charge properties (Figure 2).
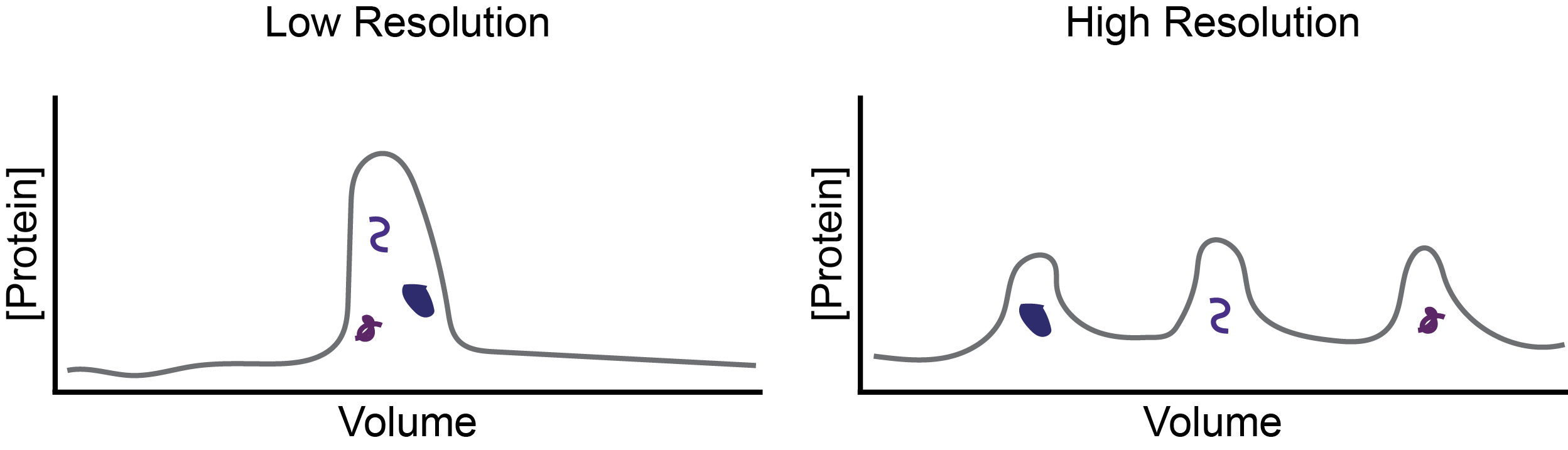
Figure 2. Resolution differences between columns determine whether proteins (different purple shapes) coelute (left) or are separated (right) during an IEX purification.
For ion exchange chromatography, resolution is primarily a function of resin bead size. The smaller the beads, the higher the resolution whereas the larger the beads the lower the resolution (Figure 3).
There is also a tradeoff between resolution and speed in that high-resolution resins are run at a lower flow rate and will therefore take longer.
Often the decision on which type of resolution is optimal depends on the exact purpose you are using ion exchange for. When it is an additional purification step between affinity tag and size exclusion purifications, it is probably fine to use moderate resolution ion exchange chromatography and enjoy the faster purification.
However, there are some special uses of ion exchange purification steps where it may be worth using high-resolution resin to purify proteins with posttranslational modifications or of different oligomeric states.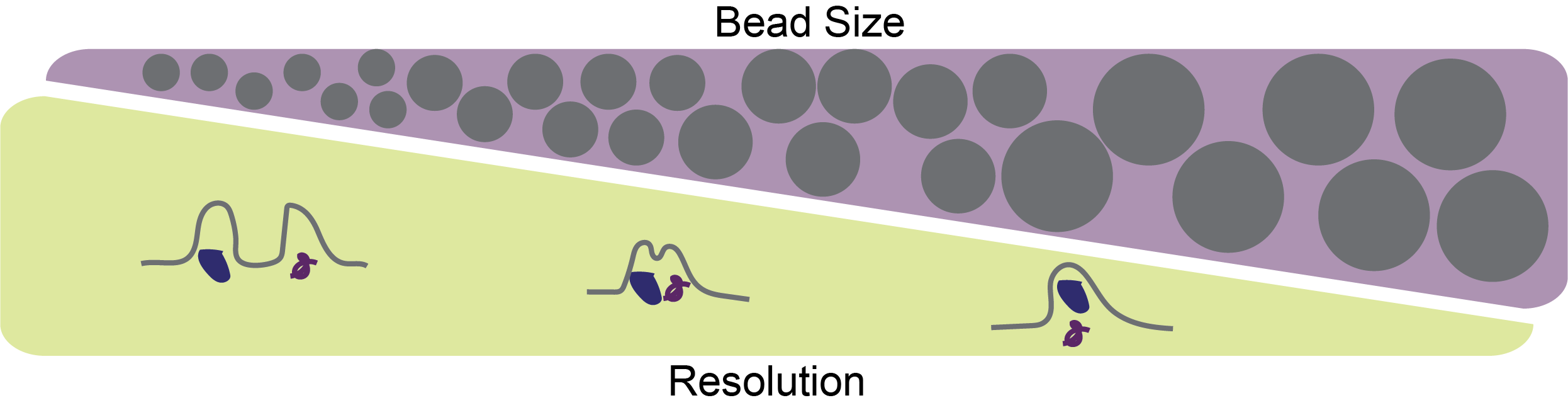
Figure 3. Smaller bead size (gray circles) leads to increased purification resolution in ion exchange chromatography.
So now you know a little bit more about the different ion exchange resin types and how to pick which resin and which resolution you want to try.

The protein streptavidin clamps onto the small molecule biotin in one of the tightest natural interactions ever discovered. Over the years, the strength of this...

Maybe you are about to start working with D-luciferin, and you begin reviewing protocols. But what you’re doing is a little different. Or what you...

Handles are common in everyday life. We use them to help us open doors, cabinets, or to hold coffee mugs, cookware, and tools. They are...

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...
