What is Glutathione Agarose?
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Glutathione is a tripeptide (glutamate – cysteine – glycine) found in many cells that plays an important role in maintaining redox balance and reducing oxidative stress (Grant, 2001). Glutathione S-transferases are a family of enzymes that facilitate glutathione’s redox maintenance function by conjugating glutathione onto oxidative substrates, thereby masking their reactivity and enhancing their secretion (Townsend et al,. 2003).
While these molecules play important roles in biology, they’re also used in biotechnology to facilitate protein purification and biochemical experiments.
Glutathione agarose is an affinity chromatography medium featuring the small molecule glutathione covalently bound to agarose beads. Glutathione binds the protein Glutathione S-transferase (GST) and GST-tagged fusion proteins enabling their purification and immobilization for pulldown experiments.
In this article, we’ll discuss what exactly glutathione agarose is, and how it’s used in protein purifications and for pulldown experiments.
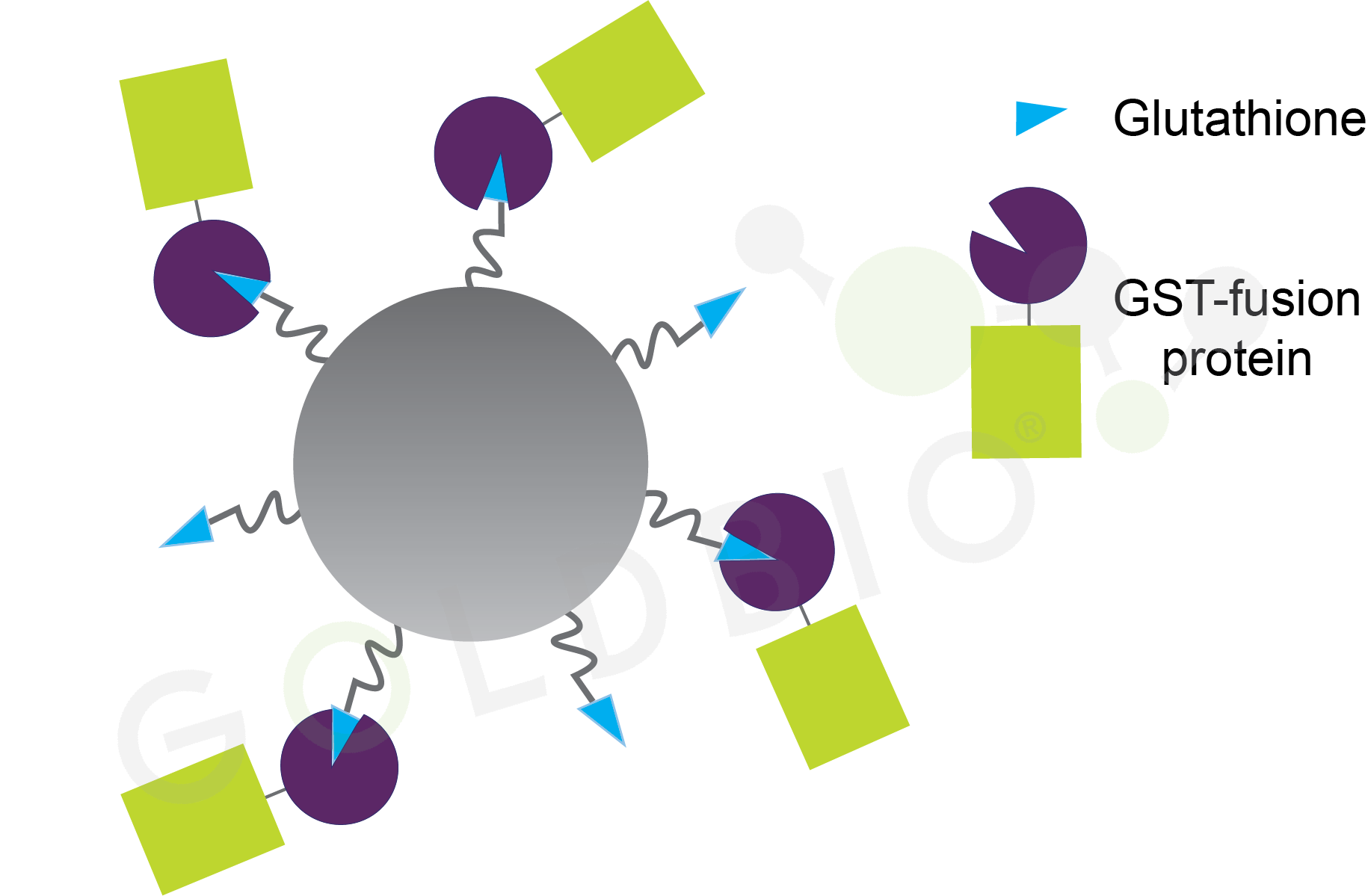
Figure 1. Glutathione agarose beads with GST-fusion proteins bound.
Glutathione agarose are agarose beads that have glutathione conjugated to them (Figure 1). Proteins that bind to glutathione, such as GST, can be purified using glutathione agarose. GST is also a commonly used solubility tag, meaning it is added N-terminally to another protein to help keep it soluble (Figure 2). Purifying GST fusion proteins is a really common use for glutathione agarose beads.
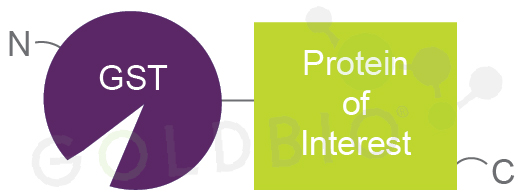
Figure 2. Cartoon
schematic of a GST-fusion protein. Fusing GST to the N-terminus of a protein of
interest helps keep it soluble.
Glutathione affinity purification, like many other types of affinity purifications, involves three key steps: 1) binding the target protein to the beads, 2) washing away contaminating proteins, and 3) eluting your protein of interest (Figure 3).
While this purification is conceptually very similar to many other types of affinity purifications, there are a few specific things to keep in mind when purifying GST-fusion proteins.
You’ll use reduced glutathione in your elution buffer to remove your GST-fusion protein from the glutathione agarose beads.
Unlike many other buffers we use for protein purification, this one is really time sensitive. You will want to add the reduced glutathione to the elution buffer right before you use the buffer.
This is because over time oxygen in the air will oxidize reduced glutathione making it worse at eluting your protein from the beads. By the way,this article has more information about the different forms of glutathione, if you're interested in learning more about that and which one to use. So that’s why you want to add the glutathione to your elution buffer no more than 1 hour in advance of when you’ll actually use the buffer.
The other thing to keep in mind is that glutathione is acidic, so when you add it to your elution buffer it will likely make the solution more acidic. So, after solubilizing glutathione you’ll want to remeasure the pH of the solution, and add concentrated base to change the pH back to your desired value.
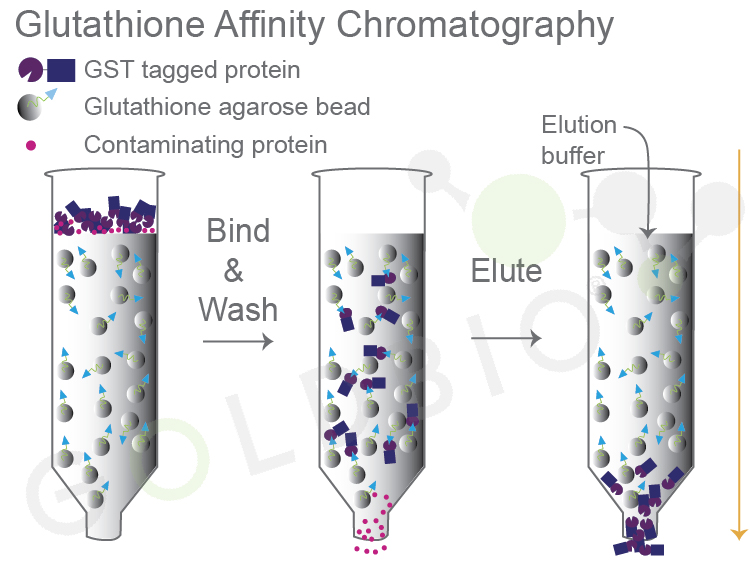
Figure 3. GST
tagged fusion proteins bind to glutathione agarose (column 2) and, after
washing, are eluted with elution buffer with excess glutathione (column 3).
GST isn’t just useful for protein purification, researchers also like to use GST as a handle to do “pull-down” experiments and see what interacts with their protein of interest. We’ve covered conceptually similar experiments with streptavidin-biotin and antibody-Protein A/G/L interactions, so check out those articles if you’re interested.
For GST pulldowns, researchers usually first purify the GST-fusion version of their protein of interest. Then they immobilize it onto glutathione beads using the same GST-glutathione interaction. Next, they’ll pour a complex biochemical mixture over the column and see which beads bind to their protein of interest (Figure 4). The biochemical mixture could be cell lysate if they’re looking to identify novel interacting partners in an unbiased way. Or it could be a purified protein, or section of a protein, that they think binds to their protein and are looking to confirm that interaction.

Figure 4. After
binding your GST-tagged protein to glutathione beads, you can use this as a
bait to search for interacting proteins.
That’s how glutathione agarose is used to facilitate the
purification of GST-fusion proteins and to investigate molecular interactions. If
you’re interested in learning more about these subjects or ready to start
purifications and experiments of your own, check out our related products and
resources below to help get you going in the lab!

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
