What is Glutathione?
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Glutathione is a tripeptide antioxidant produced by our bodies that plays a really important role in protecting our proteins and DNA from damage caused by free radicals and oxidative stress. Additionally, this versatile molecule and the proteins that bind to it are used for several purposes in biotech research.
Glutathione is a tripeptide (glutamate-cysteine-glycine) that maintains cellular redox balance and detoxification. In addition to its native roles, glutathione has several important applications in scientific research including protein purification, protein refolding, and cell culture.
In this article, we’ll discuss the cellular functions of glutathione, its different forms, and its applications in scientific research.
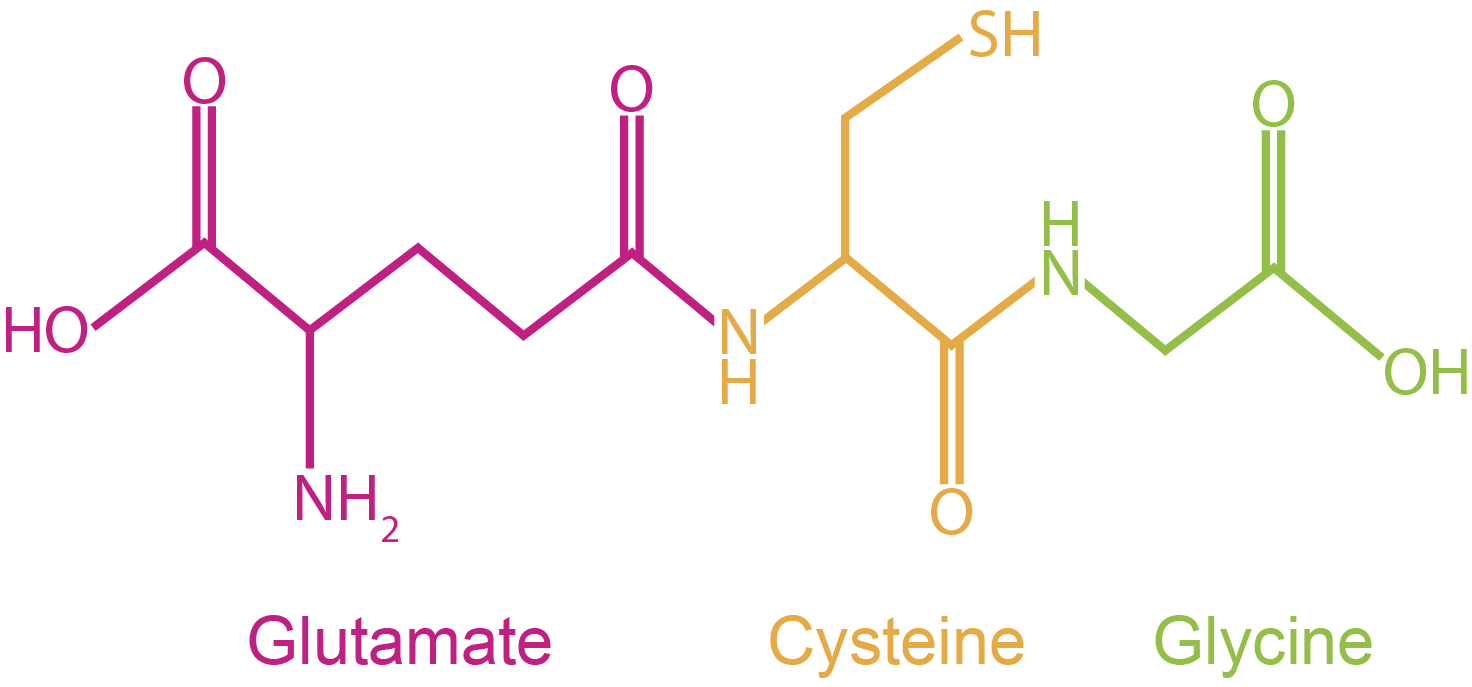
Figure 1. Reduced glutathione color coded according to its constituent amino acids: pink, glutamate; orange, cysteine; green, glycine. Note that the side chain of glutamate and the amide of cysteine are joined through a g-peptide linkage.
Why is glutathione so important?
The different forms of glutathione
Glutathione’s scientific applications
Glutathione is essential for life. In experiments, scientists have shown that mice unable to generate glutathione die before birth. Similarly, if you remove their ability to make glutathione after birth, mice will die within one month (Ashley, 2018). These results indicate that glutathione is critical for both mammalian development and homeostasis.
Why is glutathione so important? Glutathione is like the pest control of the cell. It neutralizes and clears small reactive molecules that would otherwise damage the proteins and nucleic acids that help your cells thrive. These pest molecules include oxidants and toxic metals, and we’ll discuss them more in the next section about how glutathione binds to these types of molecules.
Additionally, glutathione is an important cofactor for enzymes such as glutathione peroxidases and glutathione-S transferases (Marí et al, 2009). A cofactor is something that is required for an enzyme to catalyze a chemical reaction. But unlike reactants which are consumed by the reaction and products that are made by the reaction, cofactors remain unchanged and so they can catalyze the reaction repeatedly. These enzymes also neutralize oxidizing molecules, so glutathione has a one-two punch of directly and catalytically counteracting oxidants.
Most healthy people make plenty of glutathione on their own. However, specific patient populations such as individuals with mutations in their glutathione synthesizing enzymes, AIDS patients, and cystic fibrosis patients can benefit from glutathione supplementation (Ashley, 2018).
Ok, so we’ve covered many of the key things that glutathione does – but what exactly is glutathione?
Glutathione comes in a few different forms, and it’s good to be aware of how these different forms are used in different applications.
The molecule in Figure 1 is reduced glutathione. In redox chemistry, the reduced form of something is when it keeps its electrons and the oxidized form is when it shares them. So, in the context of glutathione, the reduced form is referring to cysteine’s sulfur atom keeping its electrons to itself (Figure 2).
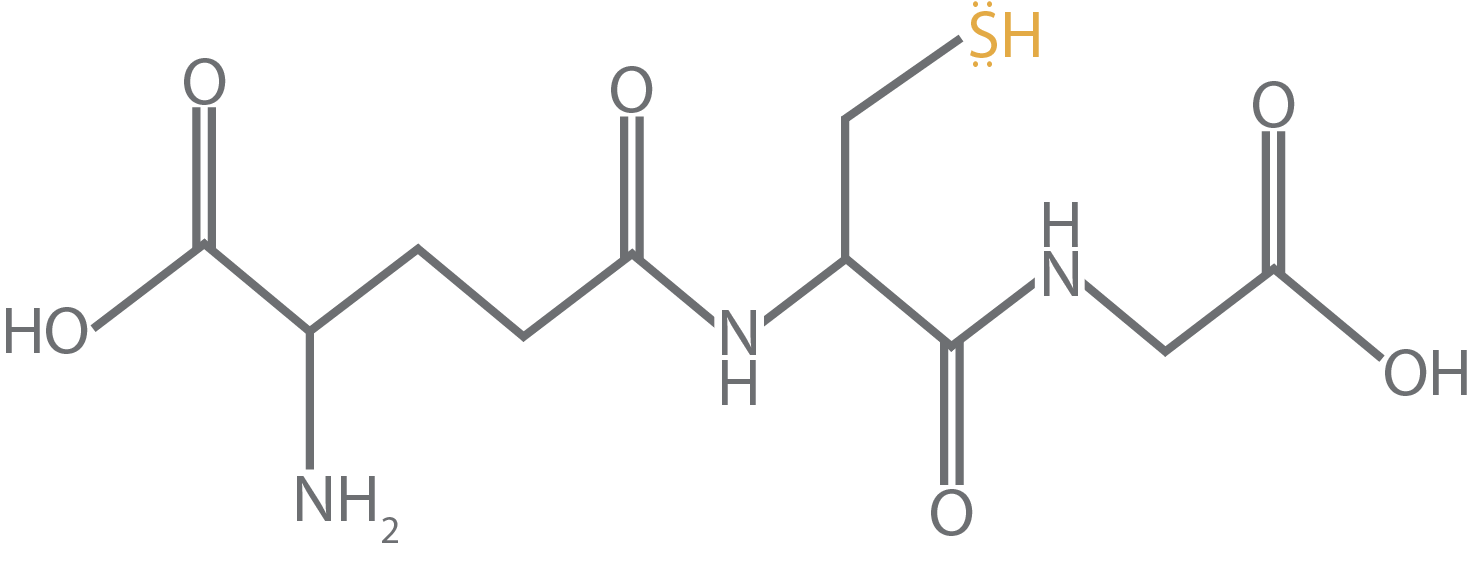
Figure 2.
Reduced glutathione with sulfur’s lone pair electrons displayed by dots. This
is the reduced form of glutathione since the sulfur atom is keeping these
electrons rather than sharing them in a covalent bond.
The oxidized form, then, is when cysteine uses those electrons to form a covalent bond with another molecule. This is how glutathione reacts with oxidants and toxic metals. So, one biological form of oxidized cysteine is when it is bound to a metal or another cellular oxidant (Figure 3).
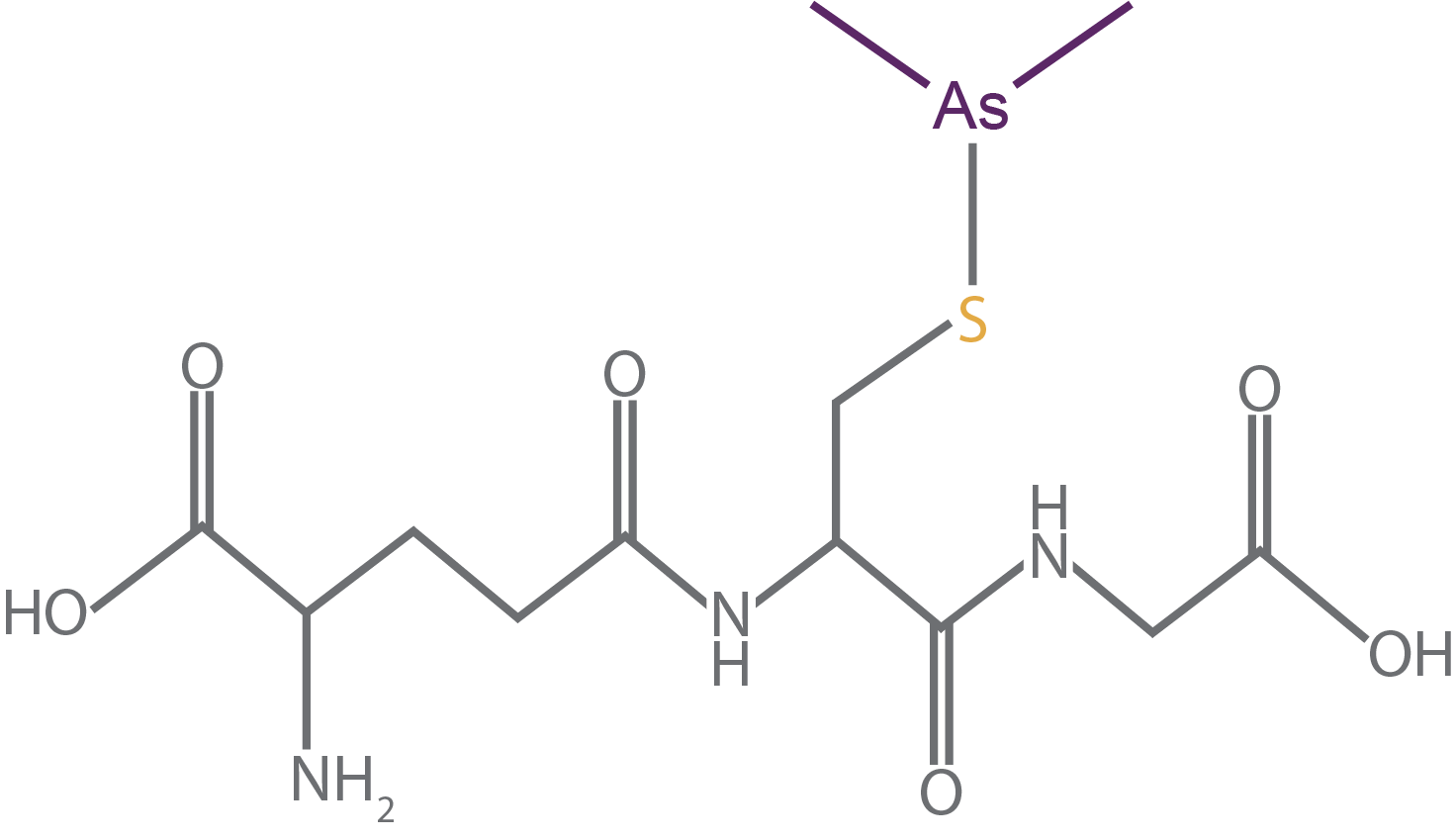
Figure 3. Oxidized glutathione bound to arsenic (purple), a human carcinogen (Rubino, 2015). Glutathione helps neutralize and clear arsenic out of cells before it can damage other biomolecules.
But when we’re talking about the oxidized form of glutathione that you can buy as a purified chemical, this is where glutathione is a dimer with the sulfur atoms from each molecule binding to one another (Figure 4).
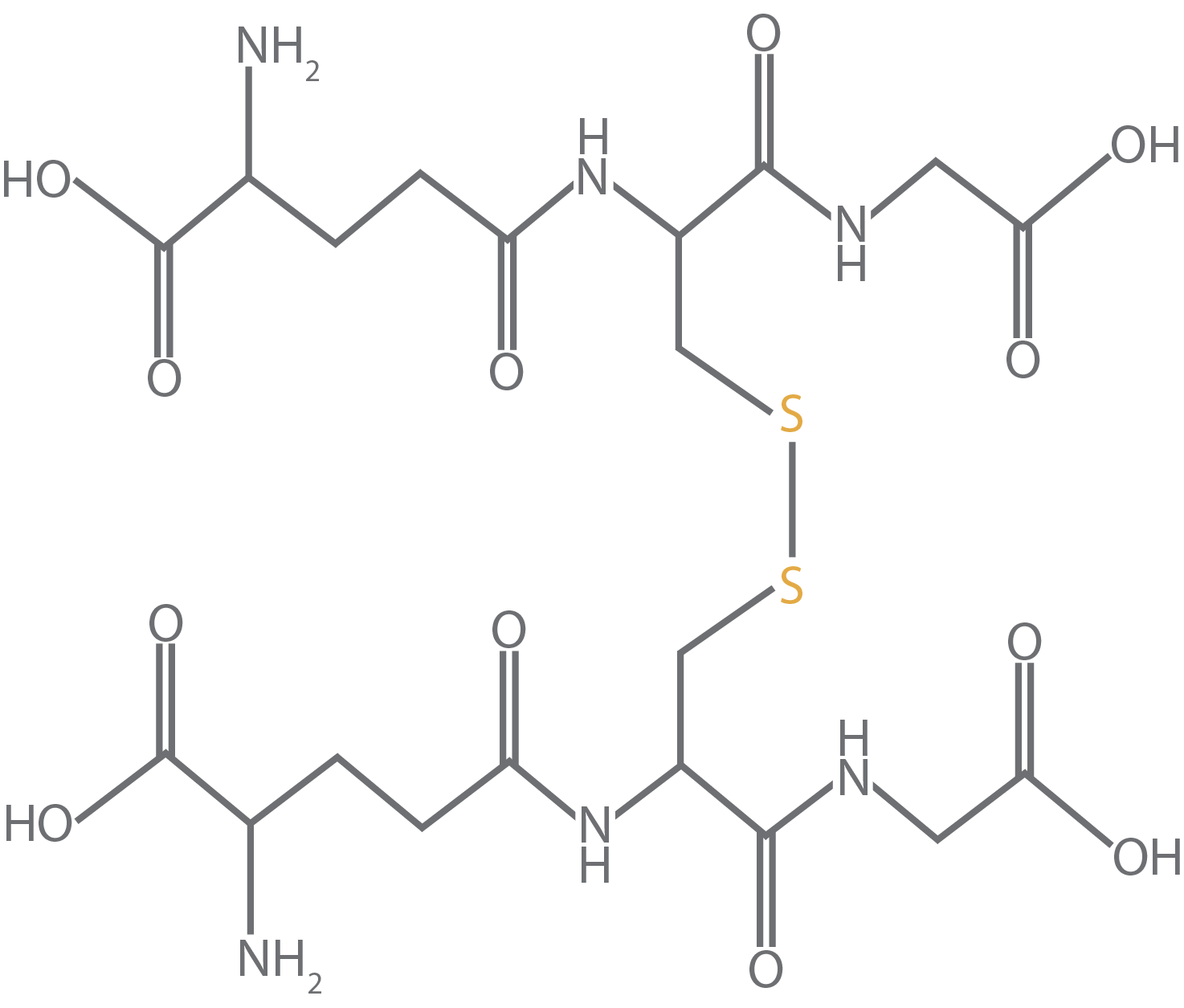
Figure 4. Oxidized
glutathione bound to another glutathione molecule.
It might sound minor, but the distinction between these forms is really important. In some of the applications we discuss in the next section, it is important to use the correct form of glutathione: reduced or oxidized.
The last form of glutathione to discuss is glutathione reduced ethyl ester. This form of glutathione has much better cellular uptake compared to either of the previous forms we discussed. So, if you’re adding glutathione to cells or animals, glutathione reduced ethyl ester is definitely the form to use. Once inside the cell, endogenous enzymes readily convert glutathione reduced ethyl ester into reduced glutathione (Levy et al, 1993).
Glutathione is used in a variety of different scientific applications:
The protein Glutathione S-transferase (GST) is a frequently used solubility tag. This means, GST is added onto other proteins to create a GST-fusion protein that will help keep the protein of interest from precipitating. Check out this article if you’re interested in learning more about solubility tags and how they work.
Since GST binds to glutathione, this interaction is used for affinity purification. The GST-fusion protein first binds to glutathione agarose, and then after other contaminating proteins have been washed away, reduced glutathione is added to elute the GST-fusion protein from the resin (Figure 5).
We have quite a few additional articles discussing glutathione agarose beads and GST affinity purification, and you can find links to those at the end of the article.
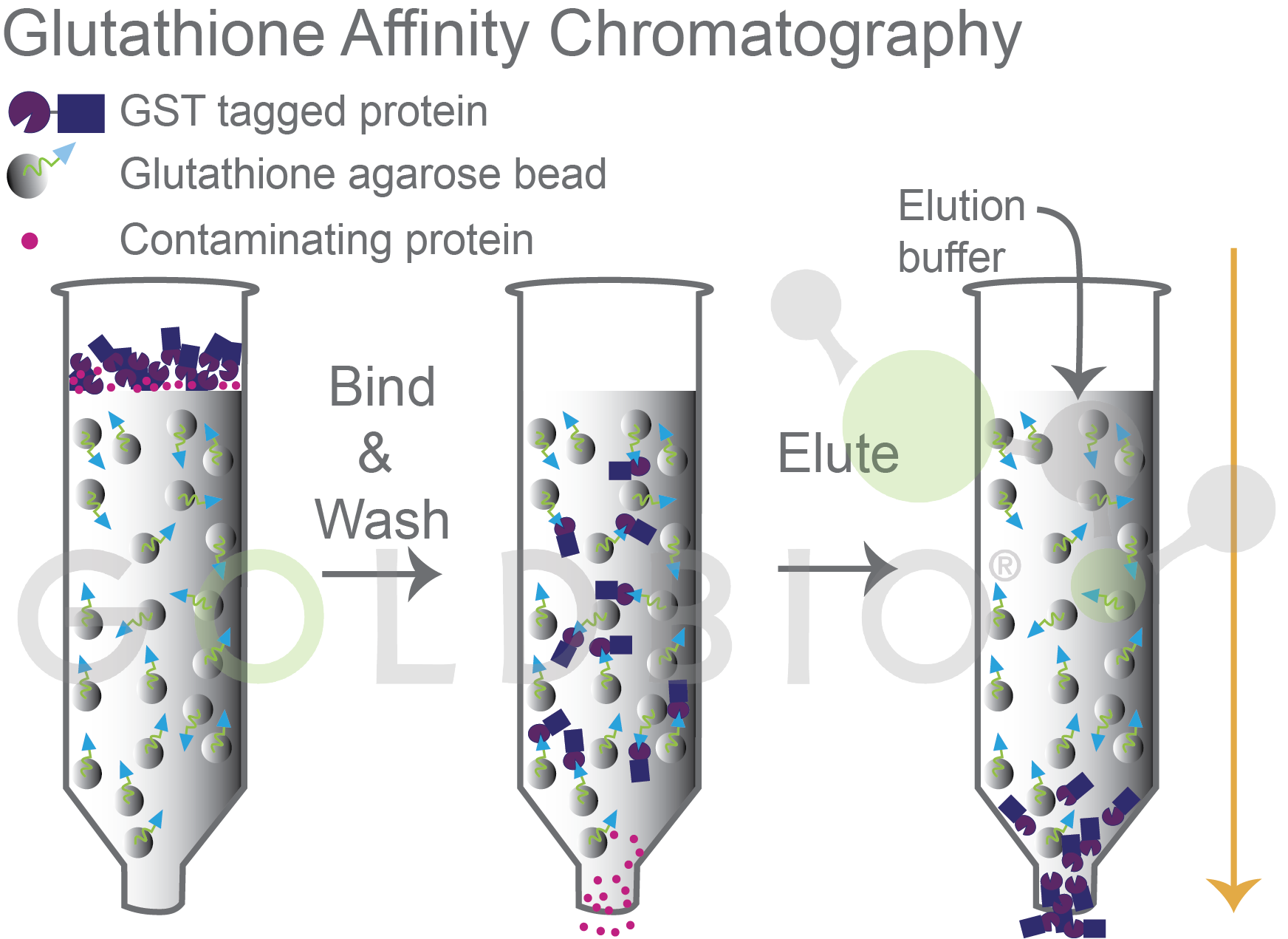
Figure 5. GST tagged fusion proteins bind to glutathione agarose and, after washing, are eluted with elution buffer with excess glutathione
Reducing agents are common additions to protein purification buffers because they help keep many proteins from forming unnatural disulfide bonds and aggregating. Disulfide bonds are covalent bonds between the sulfur atoms on cysteine side chains, such as in oxidized glutathione (Figure 4).
Commonly used reducing agents include TCEP, DTT, and bME. These reducing agents are relatively strong and do a good job preventing disulfide bond formation.
Glutathione is a bit more subtle as a reducing agent – let me explain what I mean by that. Glutathione is typically used when you want a protein to sample the different disulfide bonds it could make to help it figure out the most energetically favorable disulfide bond (Lundström-Ljung & Holmgren, 1995).
So, for example, during expression and purification of a recombinant protein, it may have a disulfide bond form, which wouldn’t normally form in the protein’s natural context. By adding a mixture of reduced and oxidized glutathione, your protein can sample different disulfide bond states and should end up in the “correct” native state, if that is the most energetically favorable (Figure 6).
Energetically favorable literally means that it has a more negative Gibbs free energy, which typically means that the protein is more compact and makes more interactions with itself, like in the rough sketch of a protein on the right side of Figure 6. In this way, glutathione can help make sure your purified protein is in the right conformation.
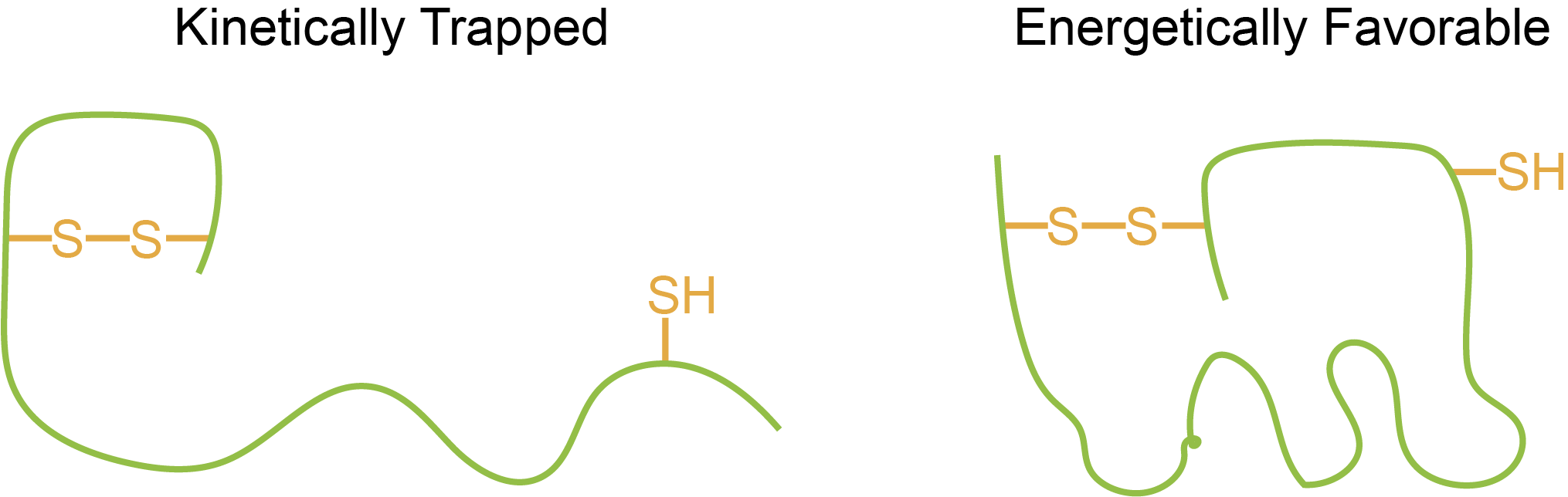
Figure 6. The left protein is a form that is kinetically trapped during heterologous expression; the first and second cysteines are disulfide bonded to each other because they were available first during protein translation. Using reduced and oxidized glutathione, you can refold this protein into a more compact and more energetically favorable protein on the right where the disulfide bond is now between the first and third cysteine residues
Glutathione is important in maintaining redox balance in cultured cells, and is already included in many formulations of cell media (Ishii & Mann, 2014). Sometimes it is desirable to add more glutathione either to media that didn’t have any, to increase its concentration in media that already has some, or to replenish old media where the glutathione may have become oxidized over time.
As we discussed above, glutathione reduced ethyl ester is definitely the form you’ll want to use for cell culture as it has a much higher cellular uptake (Levy et al, 1993).
Glutathione is more than just a small tripeptide—it’s a vital tool for life and research alike. Whether you're exploring redox biology, refining proteins, or boosting your cell culture, understanding glutathione is a smart step forward. For deeper insights or to start using it in your own experiments, explore the links below.

Covalently conjugating a small molecule to an antibody’s surface is a process called antibody “labeling.” Labeling antibodies with small molecules such as biotin or fluorophores...

DNA gel electrophoresis is one of the most widely used analytical techniques in molecular biology, providing a simple and reliable way to separate DNA fragments...

Do you have a favorite restaurant that you love because you know exactly how great the experience is going to be? There are probably a...

Using the wrong agarose can lead to smearing or poor separation. The good news is that once you know how to evaluate agarose and choose...
