Protein G Agarose Beads for Antibody Purification and Immunoprecipitation
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Protein G binds to the heavy chains of antibodies and is used to purify antibodies and support immunoprecipitation (IP) experiments. Protein G interacts with IgG antibodies from many species including human, mouse, rabbit, goat, sheep, donkey, cow and horse.
Affinity Purification of Antibodies
Protein G binds to the heavy chains of mouse and human IgG antibodies, as well as some other species such as rabbit, goat, sheep, donkey, cow, and horse (Figure 1). Protein G is useful for purifying antibodies and serving as an antibody binding site in immunoprecipitation (IP) experiments (Derrick & Wigley, 1994).By the way, check out this article if you need a quick reminder of the types of antibodies and their structure.
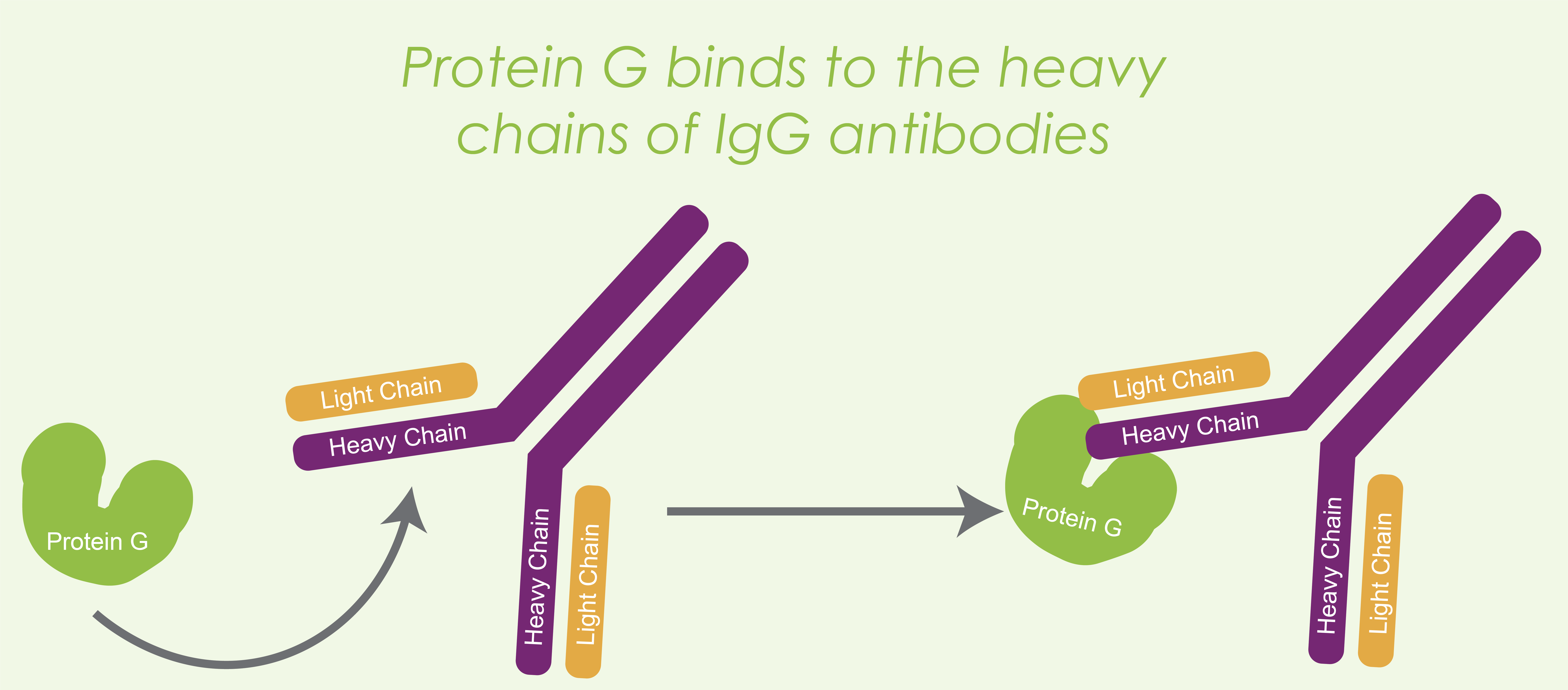
Figure 1. Protein G binds to the heavy chain of an IgG antibody.
In regard to antibody purification, antibodies are molecules that are purified out of a complex mixture such as animal serum or cell lysate. Traditionally, Protein G itself was purified from Streptococcal C or G cultures (Björck & Kronvall, 1984). However, today recombinant Protein G is frequently expressed in Escherichia coli (Goward et al, 1990).
In addition to antibodies, native Protein G also binds tightly to the protein albumin, which is highly abundant in animal serum. As you might guess, this albumin binding to Protein G impedes antibody purification out of serum. Thankfully, there is an engineered recombinant form of Protein G that still binds to antibodies, but doesn’t bind to albumin (Björck et al, 1987). So that is definitely the form you want to use if you’re purifying an antibody out of serum.
As an aside, another neat fact about Protein G is that its B domain is so soluble that it’s sometimes used as a solubility tag to keep other aggregation-prone proteins in solution (Cheng & Patel, 2004).
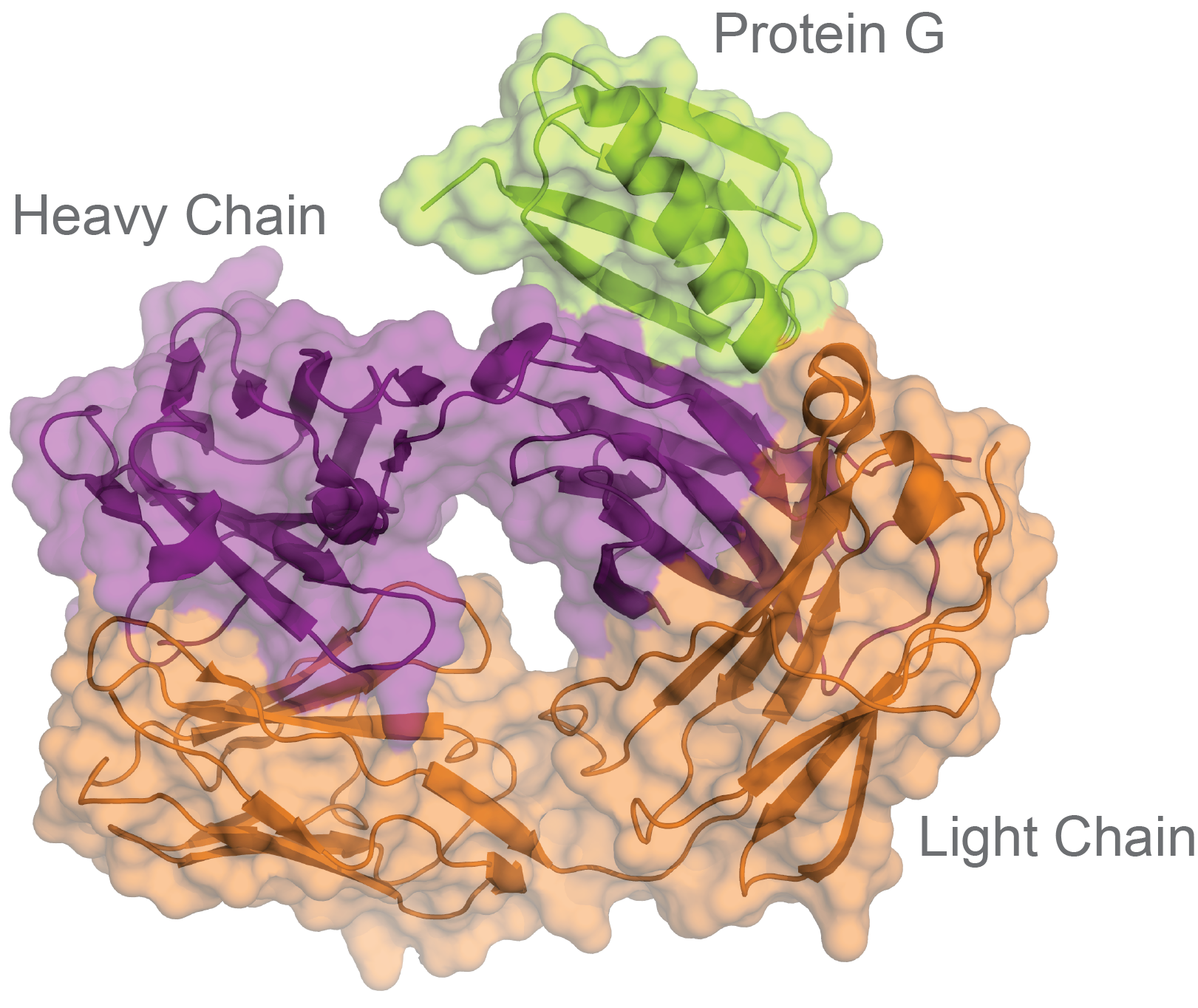
Figure 2. Protein G (green) binding to an antibody’s light chain (orange) and heavy chain (purple) (PDB: 1IGC).
Ok, back to our main topic - to purify antibodies, native or recombinant Protein G is conjugated to agarose beads. Usually, researchers just skip this step by buying Protein G agarose beads, like the kind GoldBio sells (see links at the bottom of this article). Antibody-containing solutions are then loaded onto a Protein G column. After washing, the antibodies are then eluted by adding buffer with acidic pH (pH<3) (Figure 3).
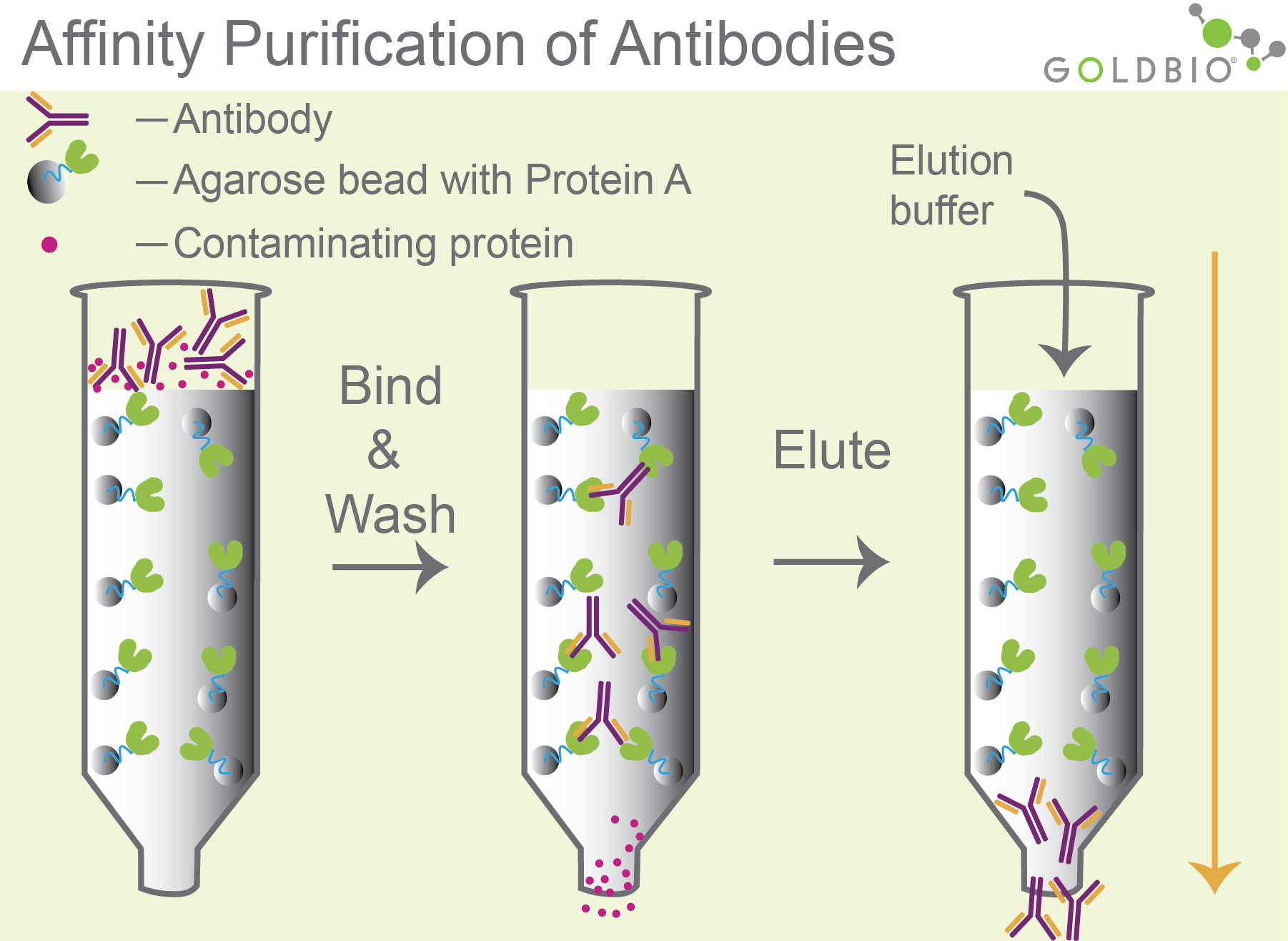
Figure 3. Antibody purification. Antibodies bind to agarose beads with Protein G (middle column). After washing (middle column), antibodies are eluted with an acidic pH elution buffer that disrupts the interaction between the antibody and Protein G (column 3).
Antibodies have poor long-term stability in acidic conditions, so the eluted antibodies are then pH adjusted back to neutral values (~ pH 7 to 8).
These antibodies can also be used to help purify other proteins in immunoprecipitation (IP) experiments.
Immunoprecipitation (IP) experiments use an antibody to bind to a particular protein and interrogate, or investigate, that protein’s molecular interactions.
Immunoprecipitation experiments work very similar to affinity chromatography, where researchers purify a protein based on a known interaction between two molecules, one of which is anchored to an agarose bead and the other fused to the molecule of interest. In this case, an antibody is bound by Protein G which is conjugated to an agarose bead, therefore being immobilized. Because antibodies are very specific for certain molecules and proteins, it’s then used to capture the protein of interest within a mix.
Protein G beads are very useful for IP experiments because they anchor the antibody. First, the antibody is loaded onto Protein G beads. Then, cell extract, or another complex biochemical mixture is loaded onto the antibody-loaded Protein G beads. That specific protein, and any of its interacting molecules, will stick to the antibody on the beads and can be eluted later. In contrast, proteins that don’t interact with the antibody-bound protein will flow through the beads before the elution (Figure 4).
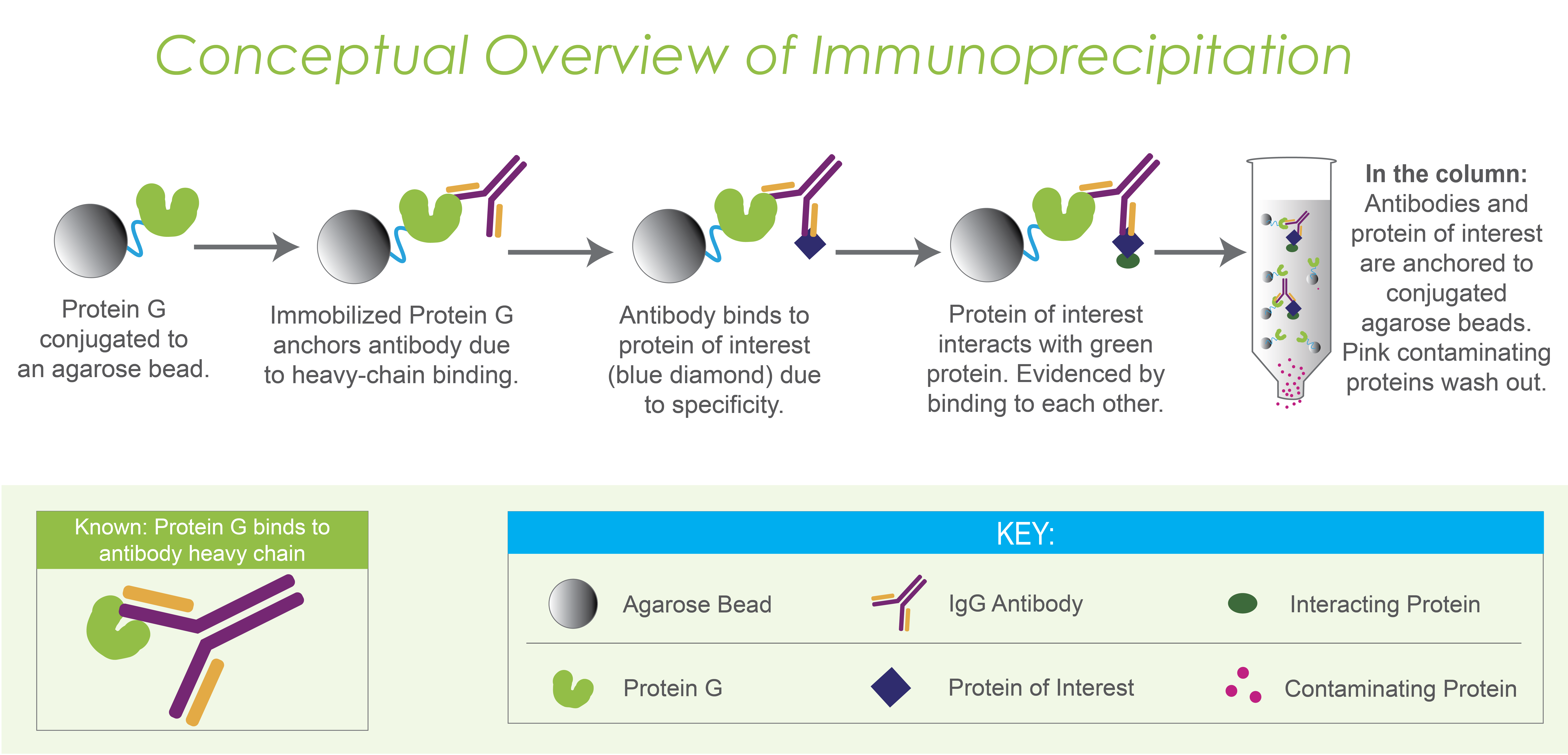
Figure 4. Immunoprecipitation. Protein G agarose beads anchor IgG antibodies (first 2 frames). The antibodies are specific for a certain antigen, and will bind to them, thereby capturing the protein of interest (blue diamond). If the protein of interest interacts with another protein (green oval), the nature of that interaction will capture those interacting proteins as well. Contaminating proteins flow through the column without binding to the antibody or protein of interest (pink circles in final frame within the column).
This type of experiment is commonly used to discover and verify molecular interactions. For more information about IP experiments and how Protein G agarose supports these foundational experiments – check out this article!
That’s a quick overview of Protein G and its function in antibody purification and immunoprecipitation experiments. See the links below for related material about Protein G, and for more information about GoldBio’s Protein G agarose products.

The protein streptavidin clamps onto the small molecule biotin in one of the tightest natural interactions ever discovered. Over the years, the strength of this...

Maybe you are about to start working with D-luciferin, and you begin reviewing protocols. But what you’re doing is a little different. Or what you...

Handles are common in everyday life. We use them to help us open doors, cabinets, or to hold coffee mugs, cookware, and tools. They are...

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...
