Troubleshooting Gel Casting/ Polymerization Issues in SDS-PAGE
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
There are three signs that indicate issues with SDS-PAGE gel casting. These include bands not being parallel, samples leaking out of the well during or after loading, and bands not separating properly during the appropriate time.
Indicators of SDS-PAGE Issues:
In this article, we will describe these observations with protein gel polymerization, and provide into what might have gone wrong. Also, for each of these situations, we will have some suggestions for troubleshooting the problem.
SDS-PAGE bands are not parallel
Samples leaking out of the well during or after loading
Bands are not separating properly despite electrophoresing for adequate time
The protein bands should appear parallel and evenly spaced in a well-executed SDS-PAGE experiment. If the gel is not cast properly or there is an issue with gel polymerization, it may result in uneven wells or slanted gel. This can cause the samples to migrate at different rates, resulting in bands that are not parallel.

Figure 1. Illustration of bands migrating at different rates with bands that are not parallel.
First troubleshooting suggestion: Before loading samples and running the gel, ensure your gel has indeed polymerized. If needed, use a higher concentration (start with one percent higher than what you had run it at) of acrylamide in your gel.
Second troubleshooting suggestion: When you pour the resolving gel, top it with a layer of isopropanol, or even water. You will see that it does not mix with your gel; rather forming a thin uniform layer on top.
When the resolving gel has solidified and you are ready to pour the mix for your stacking gel, just remove the isopropanol or water first by simply inverting the gel casting apparatus. Then pour your stacking gel mix.
This practice makes the stacking-resolving gel interface uniform – helping with coherent band separation when you run the gel.
Keeping the stacking-resolving gel interface linear across the entire gel is indeed very important. It ensures that your samples in each well start the electrophoretic separation at the same baseline. Ultimately, this helps in uniform band separation across all lanes in your gel.
Samples can leak if wells have been damaged during gel comb removal or during sample loading. This may also happen if you are using an old gel.
The result is a distorted protein gel which may show missing or uneven protein bands in the area around the well where the sample leaked.
This can result in incomplete separation and identification of the proteins of interest, or even contamination of one well’s sample with that of an adjacent well – leading to erroneous results.
So, it is important to avoid sample leakage during SDS-PAGE to ensure accurate and reliable protein separation and identification.

Figure 2. Illustration shows samples leaking out of the wells.
Troubleshooting suggestion: Extra care is required while pulling out the comb from the polymerized gel. Always remove the comb after putting the gel in the running chamber filled with running buffer.
A good practice is to fill the wells with a little bit of gel loading dye to detect any leakage prior to loading samples. Also, during loading, be careful not to touch the sides or bottom of the wells with the loading needle or pipette tips to avoid accidentally puncturing the wells.
If using precast gels, try to use ones that are not too old.
If bands are not separating properly, there might be issues with resolving gel concentration. As a result, the protein gel may appear as a smeared or blurry band instead of distinct, well-defined protein bands.
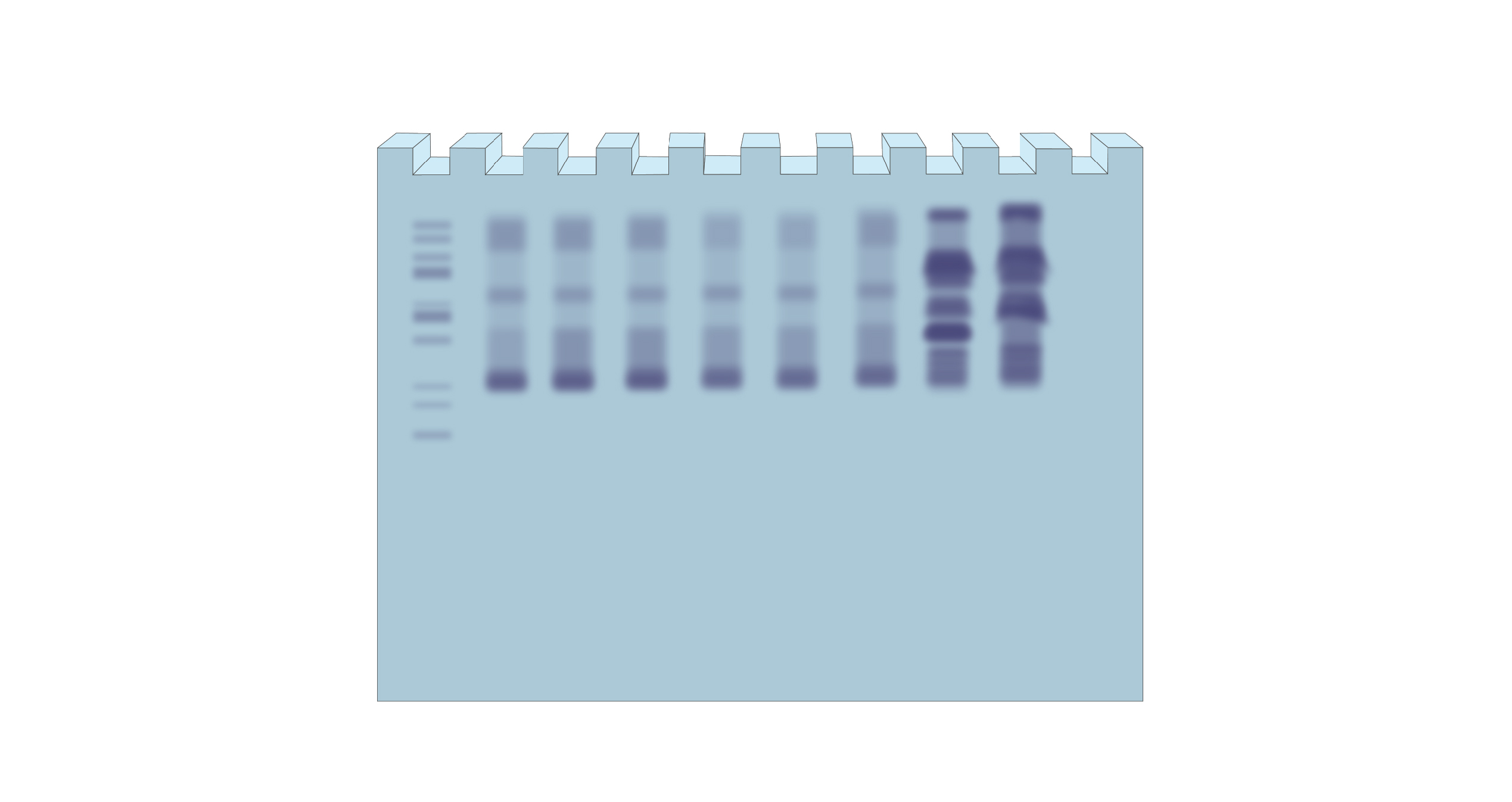
Figure 3. Illustrates protein bands not separating the way they should, resulting in a smeared or blurry gel.
Troubleshooting suggestion: Try a lower acrylamide percentage in your gel. You might need to optimize the gel pore size, especially for proteins with high molecular weights.
An electrophoresis gel, like a polyacrylamide gel in this case, is like a mesh. The protein samples pass through the pores in that mesh due to the electric current. The higher the acrylamide concentration of your gel, the smaller the pores.
For proteins with large molecular weights, you’d need bigger pores in the gel for them to migrate. Because of this, you might want a lower acrylamide percentage gel if proteins in your sample are large; or if you see them migrating very slowly even after running the gel for a long time.

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...

Covalently conjugating a small molecule to an antibody’s surface is a process called antibody “labeling.” Labeling antibodies with small molecules such as biotin or fluorophores...

DNA gel electrophoresis is one of the most widely used analytical techniques in molecular biology, providing a simple and reliable way to separate DNA fragments...

Do you have a favorite restaurant that you love because you know exactly how great the experience is going to be? There are probably a...
